Figure 2.
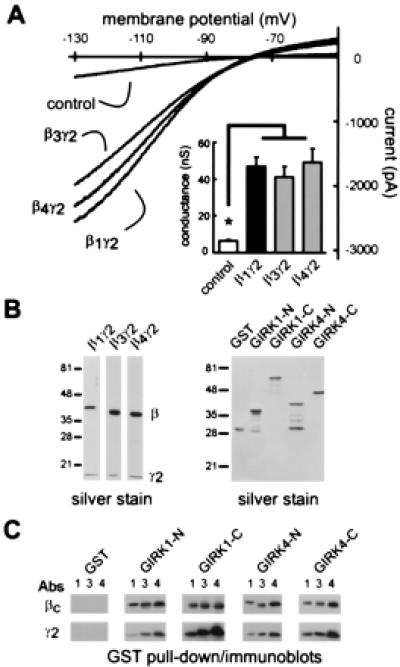
β3γ2 and β4γ2 activate GIRK channels and bind to GIRK1,4 cytoplasmic domains. (A) Sample current traces from control cells (i.e., G1,4 cells transfected with GFP alone) and G1,4 cells expressing β1γ2, β3γ2, or β4γ2; expression of these Gβγ subunits is associated with a large increase in inwardly rectifying current. (Inset) Averaged data (± SEM) show that conductance in cells expressing β3γ2 and β4γ2 is significantly higher than in control cells and comparable to that induced by β1γ2. *, Significantly different from control (P < 0.05; ANOVA with Dunnett's test). (B) Silver stain of recombinant β3γ2 and β4γ2 subunits purified from Sf9 cells (Left) and of GST fusion proteins of N- and C-termini of GIRK1 and GIRK4 isolated from bacterial cells (Right). (C) Immunoblots from GST pull down assays with Gβcommon (βc) and γ2 antisera demonstrate binding of β1 (1), β3 (3), and β4 (4), as well as γ2, to each of the GIRK-GST fusion proteins; Gβγ subunits did not bind to GST alone. Binding data are representative of two replicate experiments.
