Figure 3.
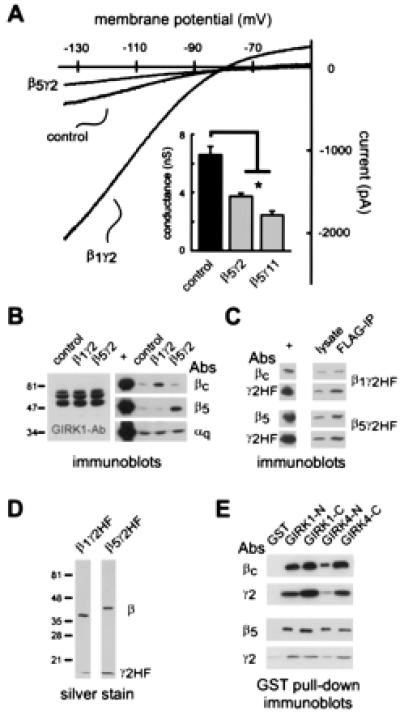
Gβ5-containing dimers inhibit basal GIRK1,4 currents and bind to GIRK1,4 channel cytoplasmic domains. (A) Sample current traces from control cells and G1,4 cells expressing either β1γ2 or β5γ2. Inwardly rectifying current was enhanced by β1γ2 but inhibited by β5γ2. (Inset) Conductance (±SEM) is decreased in cells transfected with β5γ2 or β5γ11. *, Statistically significant differences from control. *, Significantly different from control (P < 0.05; ANOVA with Dunnett's test). (B) Immunoblots of cell lysates from control cells, and cells transfected with β1γ2 or β5γ2. Overexpression of β1 (Right, Top) and β5 (Right, Middle) is apparent in the relevant transfected cells. Note, however, that there is no compensatory change in GIRK1 expression in transfected cells (Left), nor is there any change in Gαq expression (Right, Bottom). Positive controls (+) are lanes loaded with the cognate purified protein. (C) Immunoblots from G1,4 cells transfected with β1γ2HF (Top) or β5γ2HF (Bottom) demonstrate that both β1 and β5 were coprecipitated from cell lysates by using anti-FLAG antibodies, indicating that these Gβ subunits associate with γ2HF in our test system. Positive controls (+) are lanes loaded with the cognate-purified protein. (D) Silver stain of recombinant β1γ2HF and β5γ2HF subunits purified from Sf9 cells. (E) Gβ and Gγ2 immunoblots obtained after incubation of purified βγHF dimers with GIRK1,4-GST fusion proteins demonstrate that both β1γ2HF (Top) and β5γ2HF (Bottom) bind to each of the GIRK-GST fusion proteins, but not to GST alone. Binding data are representative of four replicate experiments.
