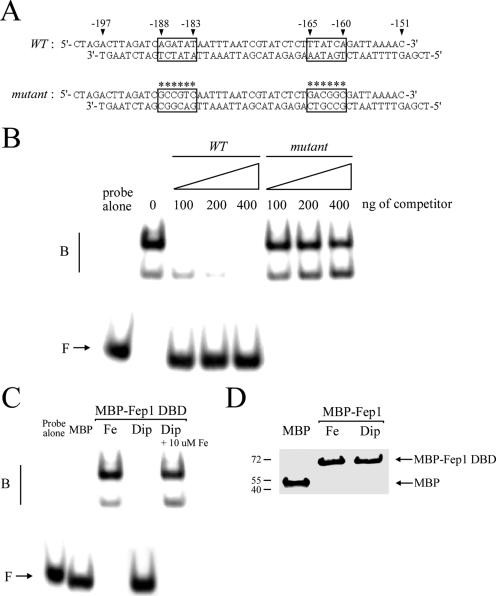
FIG. 9.
Fep1 interacts with the php4+ GATA sequences in an iron-dependent manner. (A) Sequences of the synthetic oligomers used that are derived from the php4+ promoter. The wild-type (WT) GATA elements are boxed, whereas boxes marked with asterisks indicate that each of the two GATA elements contains six substitutions (mutant). The nucleotide numbers refer to the position relative to the A of the initiator codon of the php4+ open reading frame. (B) Representative EMSA analysis using affinity-purified MBP-2Fep1241. MBP-2Fep1241 was produced and isolated from iron-treated (100 μM FeCl3) E. coli cells. Binding reactions containing the indicated amount of competitor were carried out with wild-type (WT) or mutant unlabeled oligomers. B, bound probe DNA; F, free probe DNA. (C) MBP-2Fep1241 fusion protein purified from E. coli cultures grown in the presence of 100 μM FeCl3 (Fe) or 250 μM Dip (Dip) was analyzed by gel retardation using the wild-type GATA probe. For the chelated MBP-2Fep1241 fusion peptide, its DNA-binding activity was restored by exogenous FeCl3 (10 μM Fe) (last lane). As a control, EMSA was conducted using chromatographic fractions prepared from E. coli cells expressing MBP alone. (D) Affinity-purified MBP and MBP-2Fep1241 proteins used above for panel C were analyzed by immunoblotting with anti-MBP antibody. MBP-2Fep1241 was detected with an apparent molecular mass of ∼69.5 kDa, whereas MBP fused to α peptide displays a faster electrophoretic mobility, with a molecular mass of ∼50.0 kDa.