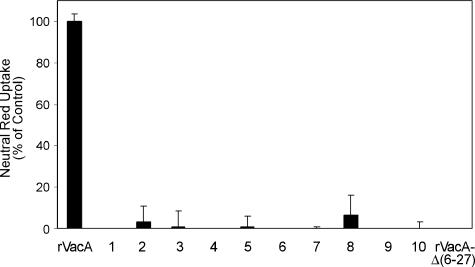
FIG. 1.
Analysis of mutant rVacA proteins that lack vacuolating activity. Plasmid pMM592, which expresses wild-type rVacA (31), was subjected to random mutagenesis by propagation in E. coli strain XL1-Red. Mutated plasmid DNA was isolated and introduced into expression strain ER2566. Soluble extracts from 4,249 individual colonies were screened for the ability to induce cytoplasmic vacuolation of HeLa cells. From this analysis, 10 mutants were isolated that failed to induce vacuolation. Results represent the vacuolating activity of soluble extracts from IPTG-induced ER2566 expressing wild-type rVacA, the 10 mutant proteins, and nonvacuolating rVacAΔ(6-27) (31, 55). Vacuolating activity was measured by neutral red uptake, and the mean and standard deviation of triplicate samples are shown (9). Results are expressed as a percentage of neutral red uptake relative to that of cells treated with wild-type rVacA.