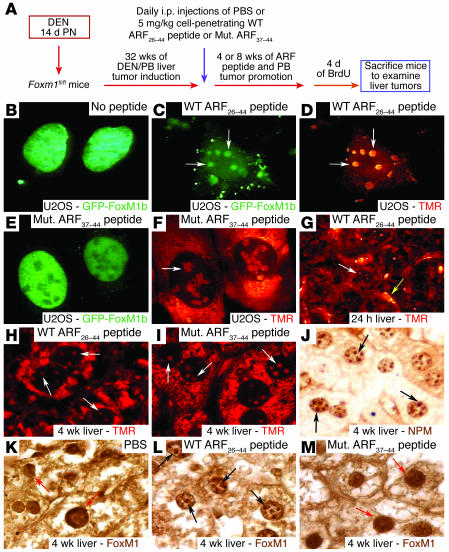
Figure 2. The WT ARF26–44 peptide targets the liver tumor FoxM1 protein to the nucleolus.
(A) Experimental design diagram of ARF peptide treatment of liver tumor–bearing mice. Liver tumors were induced in mice with DEN/PB exposure, and then they were subjected to daily i.p. injections of the WT ARF26–44 peptide or mutant ARF37–44 peptide (Mut. ARF37–44 peptide) as described in Methods. (B–F) GFP-FoxM1b protein is targeted to the nucleolus by the WT ARF26–44 peptide. U2OS cells were transfected with GFP-FoxM1b expression vector and were either left untreated or incubated for 48 hours with TMR fluorescently tagged WT ARF26–44 peptide (C and D) or mutant ARF37–44 peptide (E and F) and then analyzed for GFP (green) or peptide (red) fluorescence. (G) TMR fluorescence labeling revealed that the WT ARF26–44 peptide was localized to the hepatocyte cytoplasm and nucleolus (white arrow) and in the hepatic mesenchymal cells (yellow arrow). (H and I) Both mutant ARF37–44 peptide and WT ARF26–44 peptide are targeted to the hepatocyte cytoplasm and nucleolus (white arrows) as determined by laser confocal microscopy. Immunostaining of tumor sections with antibody specific to either NPM protein (black arrows) (J) or FoxM1 protein (K–M). WT ARF26–44 peptide targets tumor FoxM1 to the nucleolus (black arrows), whereas FoxM1 remains nuclear after treatment with mutant ARF37–44 peptide or PBS (red arrows). Magnification: ×400 (B–F and J–M); ×200 (G); and ×600 (H and I).