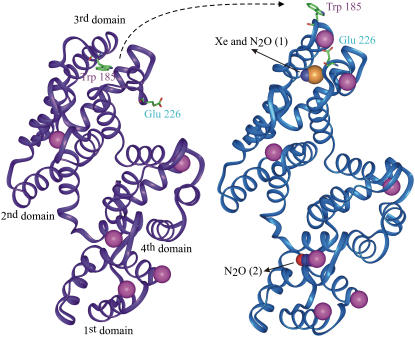
FIGURE 3.
Annexin V hinge movement and binding sites of Xe or N2O. Smooth carbon α-chain representation of annexin V monomer in low- and high-calcium conformation (colored in purple and blue, respectively) showing the hinge movement of the loop carrying the tryptophan 185 in parallel to the glutamate 226 switch. Both Xe and N2O bind within the hydrophobic cavity left vacant by the movement of the tryptophan. A second molecule of N2O binds in the center of the first domain. The color code is the same as above plus the calcium colored in pink. Figs. 2 and 3 were produced with the visualization software InsightII (Accelrys, San Diego, CA).