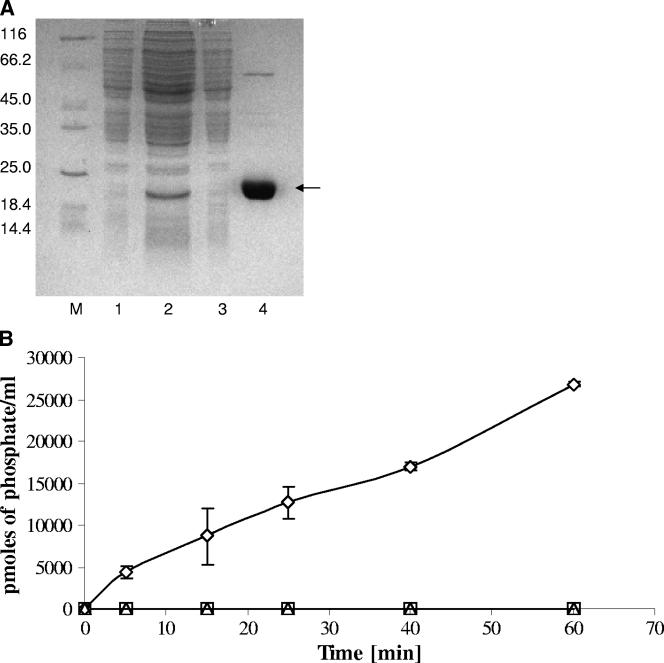
FIG. 2.
(A) Purification of the overexpressed Mab-PtpA by metal-chelation affinity chromatography. Soluble recombinant Mab-PtpA was prepared after the induction of M. smegmatis harboring the pHB-1 plasmid. Proteins were purified by using Ni-NTA resin. Aliquots of column fractions were resolved by SDS-12% PAGE and stained with Coomassie blue. Lanes: M, molecular mass marker, 1, uninduced cells (4.3 μg); 2, start material (26.6 μg); 3, wash with 20 mM imidazole (6.7 μg); 4, elution with 250 mM imidazole (8.7 μg). The protein amounts loaded in each fraction are indicated in parentheses. The molecular mass markers are indicated on the left. (B) Time-dependent dephosphorylation of O-phospho substrates. Dephosphorylation of O-phosphorylated amino acids was assayed in a time-dependent manner. The reactions were tracked by the malachite green assay using 500 μM O-phosphotyrosine (⋄), O-phosphothreonine (□), and O-phosphoserine (▵). The data represent the means of three independent experiments, and the error bars indicate the standard deviations.