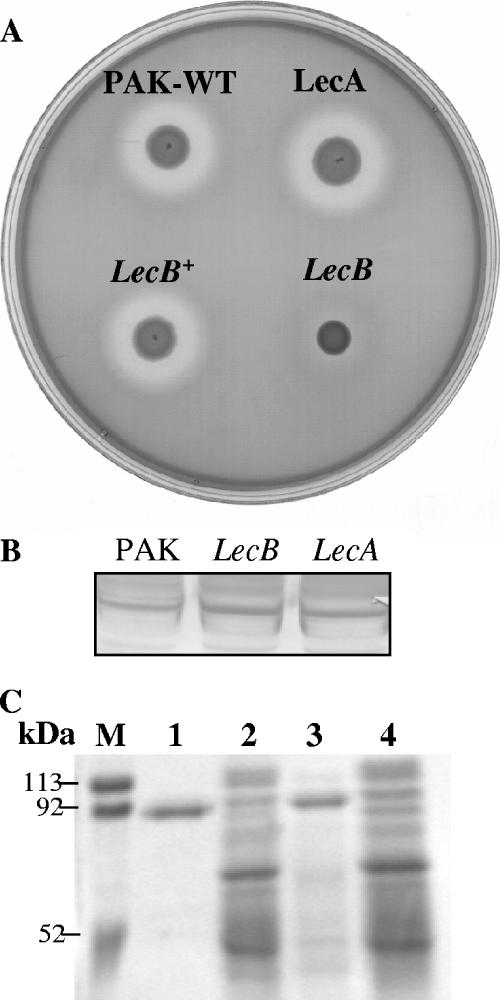
FIG. 4.
(A) Caseinolytic activity of PAK, the lecA mutant, the lecB mutant, and the complemented lecB mutant (lecB+). Strains were grown on milk-casein agar plates. The appearance of a clear zone was observed after 36 h. (B) Western blot analysis of elastase (LasB) production by wild-type PAK and the lecA and lecB mutants. LasB was detected with anti-LasB antibody and is indicative of levels of elastase in these strains. (C) Lactoferrin degradation by wild-type PAK, the lecB mutant, and the complemented lecB mutant. Lactoferrin (1 μg) was incubated with concentrated supernatants of the bacteria at 37°C for 1 h. Metalloproteases were inhibited by adding EDTA to the supernatants. Digestion products were analyzed by SDS-PAGE, and gels were stained with Coomassie blue. Molecular mass markers are shown. Lane 1, lactoferrin (∼90 kDa); lane 2, wild-type PAK; lane 3, lecB mutant; lane 4, complemented lecB strain.