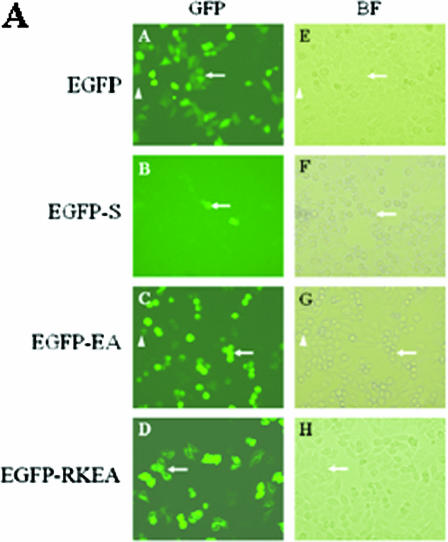
FIG. 4.
(A) Transient expression of EGFP-ExoS in HeLa cells. HeLa cells were transfected with EGFP fusion protein constructs as indicated. HeLa cells were observed under a microscope using GFP fluorescence (A to D) or a bright-field (BF) filter (E to H) at 16 h posttransfection. A and E, EGFP vector control; B and F, EGFP-S, wild-type exoS in fusion construct; C and G, EGFP-EA, exoS(E381A) in fusion construct; D and H, EGFP-RKEA, exoS(R146K) in fusion construct. In each pair of images, the arrows refer to the same cells with detectable GFP. (B) FACS analysis of GFP signals in HeLa cells transiently transfected with fusion constructs. HeLa cells were collected at 24 h posttransfection and subjected to flow cytometry analysis using FL-1 channel on a FACSort cytometer. Each color-coded line on the histogram represents a collection of cells that were transfected with an indicated construct, with the GFP intensity of each cell on the x axis and counts of the cells with the same intensity on the y axis. A threshold intensity for a GFP-positive cell (GFP+ in the figure) was set based on a vector control, and the percentages for GFP-positive cells, of the total, are presented on the right. CTRL means no plasmid DNA was added for transfection. The results are from a single experiment that is representative of three replicated experiments. (C) Cytosolic localization of fusion proteins in HeLa cells after transient transfection with fusion protein expression plasmids. HeLa cells were transfected with EGFP fusion protein constructs as indicated. The resulting HeLa cells were fixed for 16 h after transfection and then stained with DAPI and observed under a phase-contrast DMIRB inverted fluorescence microscope (Leica, Germany) using a GFP fluorescence (a and b) or UV (DAPI, c and d) filter; merged images of GFP and DAPI are also shown (e and f). Panels a, c, and e show EGFP-EA [exoS(E381A)] and panels b, c, and f show EGFP-RKEA [exoS(R146K/E381A)] in fusion constructs.