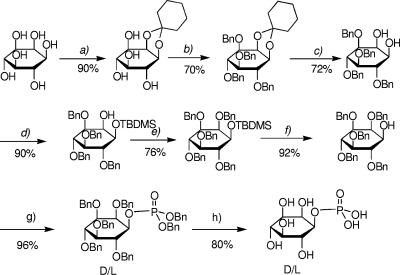
FIG. 1.
Scheme for the chemical synthesis of dl-myo-inositol 1-phosphate from myo-inositol. (a) (i) Cyclohexanone (16 eq), DMF-toluene, p-TsOH, 6 h, 150°C; (ii) EtOH, p-TsOH, 1 h, room temperature. (b) BnBr (12 eq), DMF, 16 h, room temperature. (c) TFA (7 eq), MeOH (1.2 eq), DCM. (d) TBDMSOTf (1.2 eq), (i-Pr)2Net (1.2 eq), DCM. (e) BnBr (4 eq), NaH (4 eq), DMF, 2 h, room temperature. (f) 1 M TBAF (1.5 eq), THF, 2 h, room temperature. (g) (i) (BnO)2PN(iPr)2 (1.5 eq), 1H-tetrazole (1.5 eq), DCM, 2 h 30 m, room temperature; (ii) MCPBA (2 eq), DCM, 1 h 30 m, −40°C to 0°C. (h) Pd-C, 20 lb/in2 H2, EtOH, 2 h, room temperature. The yields for the individual steps are indicated as percentages.