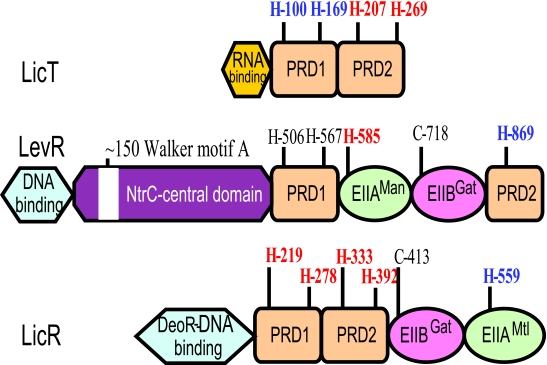
FIG. 7.
Domain structure of the transcription antiterminator LicT and the transcription activators LicR and LevR of B. subtilis. The N-terminal RNA or DNA binding domain and the two regulatory domains PRD1 and PRD2 together with their conserved histidyl residues are indicated. Histidyl residues, which have been shown to become phosphorylated, are in boldface type. When the phosphorylation exerts a positive effect on the activity of the regulator, they are shown in red, whereas blue indicates the sites of negative regulation. For LicT, its four conserved histidyl residues become phosphorylated. Phosphorylation in PRD1 inhibits, while phosphorylation in PRD2 stimulates, LicT activity. Most other antiterminators of the BglG/SacY family also contain four conserved histidines, but sometimes, not all of them can be phosphorylated. LevR contains an NtrC-like central domain and EIIAMan- and EIIBGat-like domains inserted between the complete PRD1 and a truncated PRD2. The positive site of phosphorylation is His-585, and the negative site is His-869. The conserved histidyl residues His-506 and His-567 in PRD1 and the EIIBGat phosphorylation site (Cys-718) do not seem to become phosphorylated. An identical organization can be observed in most other NifA/NtrC-type PRD-containing transcription activators. However, a few LevR-like regulators contain a complete PRD2 with an additional potentially phosphorylatable histidyl residue. The DNA binding domain of LicR resembles that of DeoR, and PRD1 and PRD2 are followed by an EIIBGat-like and EIIAMtl-like domain. An identical domain organization can be observed in other DeoR-type PRD-containing transcription activators. All four conserved histidines in PRD1 and PRD2 are sites of positive regulation, while LicR activity is inhibited by phosphorylation at His-559 in the EIIAMtl-like domain.