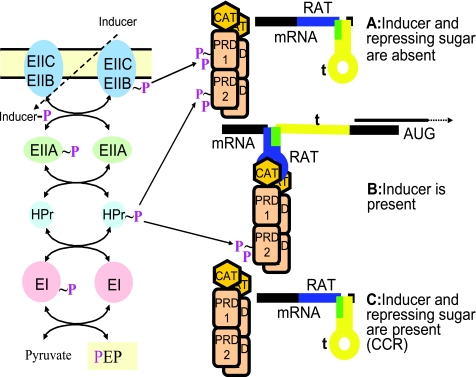
FIG. 8.
Transcription regulation by the PTS via PRD-containing transcription antiterminators. (A) In the absence of the corresponding inducer, full-length transcription of several PTS-encoding genes/operons is inhibited owing to the formation of a terminator structure (t, yellow) on the nascent mRNA upstream from the start codon. Under these conditions, the corresponding antiterminator cannot bind to its RNA target, RAT (blue), because the EIIB is mainly phosphorylated and transfers its phosphoryl group to PRD1 of the antiterminator. The absence of a repressing sugar is expected to also allow phosphorylation at the activating domain (PRD2) by P∼His-HPr. However, the negative effect of phosphorylation at PRD1 is dominant. The RAT sequence can also form a stem-loop, which, however, was calculated to free less energy than the terminator t. Interestingly, in most antiterminator-controlled PTS operons, the two sites RAT and t overlap (green), and the formation of the terminator therefore prevents the formation of the RAT stem-loop and vice versa. (B) If an inducer is present, the EIIB as well as PRD1 of the corresponding antiterminator will be present mainly in an unphosphorylated form. Because antiterminator-controlled PTSs are usually low-capacity sugar transporters, there will be sufficient P∼His-HPr to guarantee activating phosphorylation in PRD2. The activated antiterminator binds to its RAT and thus favors the formation of the RAT stem-loop, thereby preventing the formation of the terminator stem-loop, as part of it (in green) is already used for the RAT stem-loop. (C) If, in addition to the inducing sugar, a repressing carbohydrate is present, the amount of P∼His-HPr will be low in the cells. In firmicutes, P-Ser-HPr will also be formed, which further lowers the amount of P∼His-HPr. These conditions prevent activating phosphorylation at PRD2, and most antiterminators are therefore inactive, although the presence of the inducer probably prevents the phosphorylation in PRD1. Dephosphorylation of PRD2 in the presence of a rapidly metabolizable PTS sugar therefore represents a CcpA-independent, P-Ser-HPr-dependent CCR mechanism. ptsH1 as well as licT(Pia) but not ccpA mutants are relieved from this type of CCR.