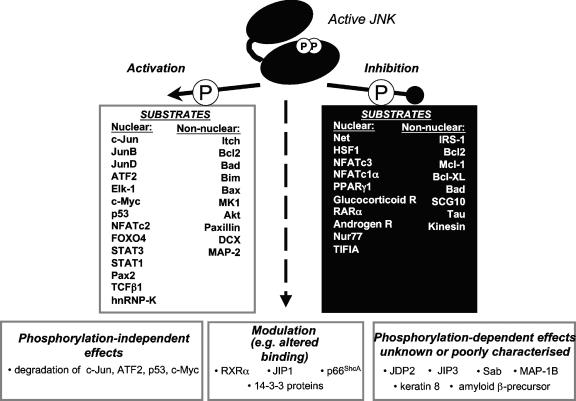
FIG. 3.
Summary of the substrates of JNKs discussed in this review. Following its activation by phosphorylation of a specific threonine and tyrosine within its activation loop, JNK can phosphorylate a range of substrates. Phosphorylation can modulate the substrate protein activity in a positive or negative fashion; JNK binding can even modulate the activity in a phosphorylation-independent manner. In some cases, the consequences of phosphorylation by JNK have not yet been defined. The mitochondrial protein Bcl2 is shown as a protein that is both activated and inhibited following its phosphorylation by JNK because currently there is evidence to support either effect (76, 146, 331). Similarly, JNK actions on Bad have been shown to both increase and decrease its activity. In this example, these divergent effects have been attributed to the ability of JNK to phosphorylate distinct sites (84, 344), although the effects of JNK-mediated phosphorylation of Bad Ser128 to increase its proapoptotic actions have been questioned (347).