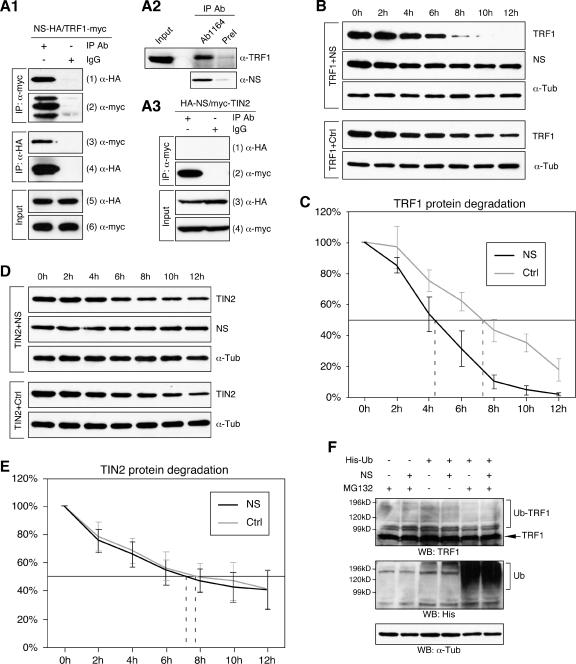
FIG. 9.
NS interacted with TRF1 and negatively regulated its protein stability. (A) The interaction between NS and TRF1 was shown by in vivo coimmunoprecipitation assays. HEK293 cells were cotransfected with HA-tagged NS and myc-tagged TRF1 and immunoprecipitated with the anti-myc antibody (first and second rows, left columns), anti-HA antibody (third and fourth rows, left columns), or mouse immunoglobulin G (first to fourth rows, right columns). The copurified proteins (first and third rows) and the immunoprecipitates (second and fourth rows) were detected by Western analyses using the indicated antibodies. (A2) The endogenous TRF1 could be copurified with the endogenous NS in HEK293 cells immunoprecipitated by the anti-NS antiserum (Ab1164) but not by the preimmune serum (PreI). The NS immunoprecipitates are analyzed in the bottom panel. (A3) NS failed to bind a TRF1-interaction protein, TIN2, in coimmunoprecipitation assays in which HEK293 cells were transfected with HA-tagged NS and myc-tagged TIN2 and immunoprecipitated with the anti-myc antibody. (B) The effect of NS on the TRF1 protein stability was examined by a protein degradation assay in which myc-tagged TRF1 and HA-tagged NS (or control vector, Ctrl) were coexpressed in HEK293 cells. Thirty-six hours after transfection, living cells were treated with cycloheximide for 0 to 12 h at 2-h intervals. The amounts of TRF1 and NS proteins were determined by anti-myc and anti-HA Western blots, respectively. Anti-α-tubulin (α-Tub) Western blots were used as loading controls. (C) The amounts of TRF1 protein at every time point were measured quantitatively from scanned images of four protein degradation experiments using the ImageJ 1.36b software and expressed as a percentage of the TRF1 protein amount at the 0-h time point. (D, E) Coexpression of NS did not alter the TIN2 protein stability examined by the same assay. The amounts of TIN2 and NS proteins were determined by anti-myc and anti-HA Western blots. Anti-α-tubulin (α-Tub) immunoblots were used as loading controls. The amounts of TIN2 protein at every time point were expressed as a percentage of the TIN2 protein amount at the 0-h time point. (F) The NS effect on the ubiquitination of TRF1 protein was measured in vivo by expressing His-tagged ubiquitin (His-Ub) and NS (or control plasmid) in HEK293 cells treated with a proteasome inhibitor MG132. TRF1 protein was detected by anti-TRF1 Western blots. Lysates were blotted with the anti-His antibody to show the ubiquitination effect (Ub) and the anti-α-Tub antibody for loading controls. Coexpression of NS did not increase the high-molecular-weight species of TRF1 (Ub-TRF1) compared to the control samples.