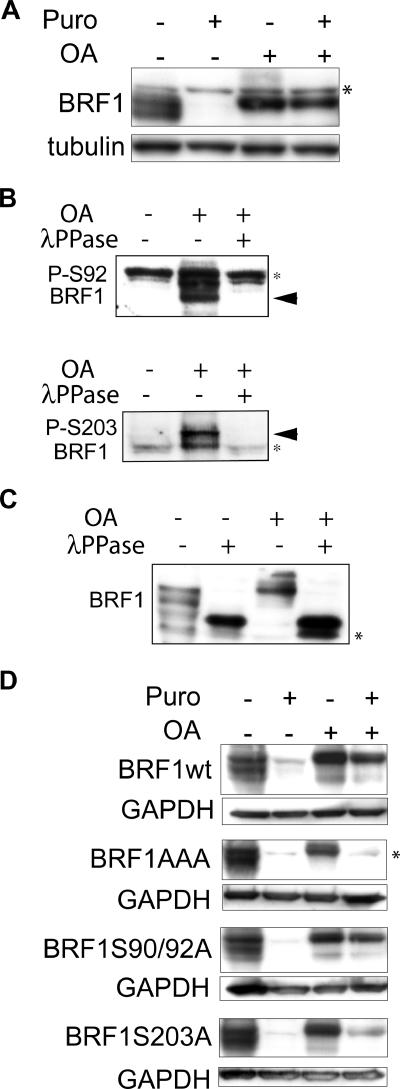
FIG. 5.
Phosphorylated BRF1 is stable. (A) HT1080 cells were treated with puromycin (Puro; 50 μg/ml) and OA (400 nM) either alone or in combination for 6 h, and lysates were probed with an anti-BRF1 antibody. The persistence of BRF1 in OA-induced cells, even when translation is blocked, indicates that it is stabilized by phosphorylation. Note the upward shift in BRF1 migration characteristic of the hyperphosphorylated form. (B) Probing of lysates from OA-induced cells (400 nM, 3 h) with phosphospecific antibodies directed against phospho-Ser92 and phospho-Ser203 confirms that these sites are phosphorylated in HT1080 cells. Phosphospecific signals (arrowheads) are detected after OA treatment and are sensitive to λ phosphatase (λPPase). (C) Lysates from panel B were probed with the anti-BRF1 antibody. The various BRF1 bands are shifted upward after OA treatment and revert to a single high-mobility band after dephosphorylation by λ phosphatase. (D) A series of BRF1 phospho-null mutants were stably expressed in slowC cells. Translation arrest and OA-induced phosphorylation of BRF1 were performed as described for panel A. Constitutive lability of BRF1AAA protein despite OA-induced hyperphosphorylation suggests that these sites are critical for phosphorylation-dependent stabilization. Unlike the endogenous protein, transfected BRF1 migrates slightly higher than the nonspecific band after OA treatment (compare with panel A) because of the presence of His and Xpress tags. Asterisks indicate nonspecific bands.