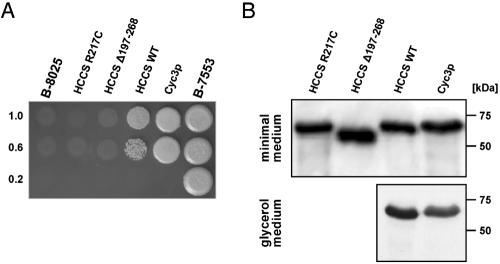
Figure 4. .
HCCS mutant proteins are not able to complement S. cerevisiae CYC3 deficiency. A, Functional complementation of the S. cerevisiae strain B-8025. B-8025 was transformed with human wild-type HCCS (HCCS WT), the mutants Δ197–268 and R217C, or yeast CYC3 (Cyc3p) expression constructs and was grown on minimal medium. Transformants were grown in liquid minimal medium, and aliquots of 5 μl of saturated and diluted cultures were spotted on glycerol medium containing copper (to induce expression of GST-fusion proteins) and were incubated for 5 d at 30°C. The top row shows spots of saturated cultures, and the middle and bottom rows show spots of dilutions; dilution rates are indicated to the left of the figure. Note partial restoration of growth by Cyc3p and wild-type HCCS, whereas no growth was observed for the untransformed strain or that expressing HCCS Δ197–268 or HCCS R217C. In parallel, all strains were also spotted on plates with glucose-containing minimal medium and showed normal growth (data not shown). Strain B-7553 served as wild-type growth control. B, Expression of GST-HCCS–fusion proteins determined by western blotting. Expression of GST-HCCS R217C–, Δ197–268–, wild-type–, and GST-Cyc3p–fusion proteins in yeast strain B-8025, grown in minimal medium with copper, was demonstrated by immunoblotting (top panel), whereas, in strain B-8025 grown in glycerol-containing medium, only GST-HCCS wild-type– and GST-Cyc3p–fusion proteins were expressed (lower panel). B-8025 transformed with the GST-HCCS Δ197–268 or GST-HCCS R217C construct did not grow under this condition.