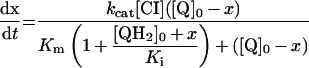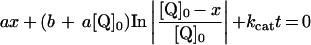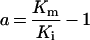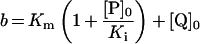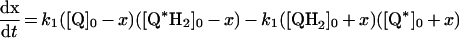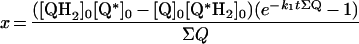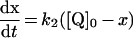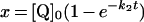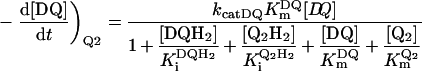Table 3. Rate equations describing the four reactions shown in Scheme 2.
x is the amount of the starting material that has reacted to form products after time t and [A]0 is the concentration of species A at time zero. In (D), only the rate equation for the decrease in DQ at constant Q2 concentration is given; an analogous equation is readily derived for Q2.
| Rate equation | Integrated rate equation | |||
|---|---|---|---|---|
| A. Catalysis: single Q with product inhibition |
|
|
||
|
||||
|
||||
| B. Exchange reaction |
|
|
||
| ∑Q is the sum of the concentrations of all the Q and QH2 species. | ||||
| C. Inhibitor-insensitive reaction |
|
|
||
| D. Catalysis: two Qs with product inhibition |
|