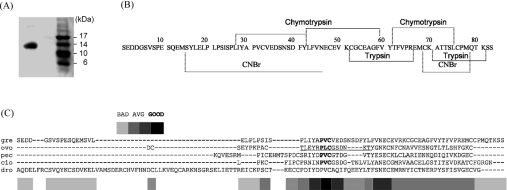
Figure 1. Purification of greglin.
(A) SDS gel electrophoresis of purified greglin after ion-exchange chromatography. (B) Complete amino acid sequence of greglin obtained from N-terminal sequencing of overlapping peptides produced by chemical and proteolytic cleavages followed by RP-HPLC separation of each greglin isoform after reduction and alkylation. Proteolytically cleaved and chemically cleaved fragments are underlined. (C) Alignment of the sequences of greglin (gre) and Kazal-related homologues calculated using T-coffee [45] [OMTKY-III (ovo), porcine PEC-60 (pec), C. intestinalis (cio) and D. melanogaster (dro)]. The inhibitory site of OMTKY-III (P4 to P3′) is underlined. The scores resulting from the multiple sequence analysis are identified as bad, average (AVG) and good.