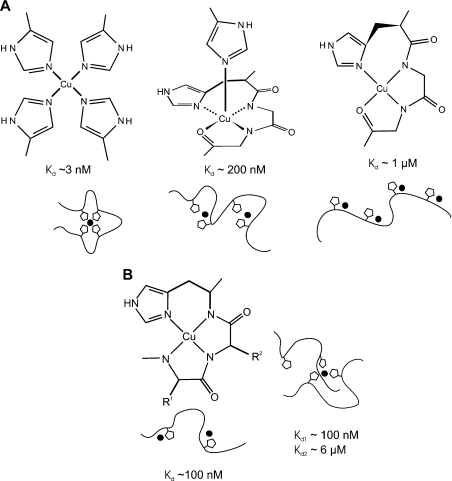
Figure 9. Multiple modes of copper binding in PrP.
(A) At least three different modes of copper (II) co-ordination occur within the octapeptide repeats at pH 7.4. Models for the structure of each mode of binding based on our data and those of others are shown, alongside estimates for the dissociation constants. The cartoons represent the peptide backbone in the peptide PrP57–91, with the histidine side chains shown as pentagons and the copper (II) ion represented by the black circle. (B) The structure for the Cu(PrP91–115) and Cu2(PrP91–115) complexes is shown as described in the literature (left). At present, there are insufficient data to propose a detailed structure for the Cu(PrP91–115)2 complex, although our NMR data indicate that either two or three of the available histidine residues are involved in co-ordinating the metal ion (right). Estimates of the dissociation constants are given for all the complexes based on our measurements with the exception of the lowest binding affinity shown in (A) which is based on reports in the literature. The cartoons represent the peptide backbone and the side chains of His96 and His111. At present, it is not possible to distinguish whether either histidine is favoured for any particular co-ordination position.