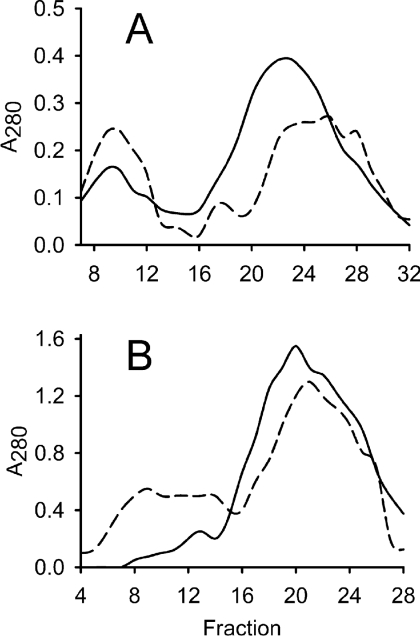
Figure 2. Gel filtration of affinity-purified H-subunit.
Elution of protein was followed by absorbance (A) at 280 nm. The column was calibrated with apoferritin (443 kDa, peak at fraction 9), β-amylase (200 kDa, fraction 18), alcohol dehydrogenase (150 kDa, fraction 22) and BSA (66 kDa, fraction 29). (A) Affinity-purified H-subunit (40 mg) was exposed to light before loading on to a Sephacryl S-200 HR 16/60 column (dashed line). An equal sample, kept in darkness, was used as a control (solid line). The first (fractions 7–12) and second (fractions 18–27) peaks of each analysis were selected to represent the high- and low-molecular-mass fractions respectively and used in further analysis. (B) Pooled and concentrated fractions from low-molecular-mass H-subunit obtained by gel filtration were exposed to light and a second round of gel filtration was performed (dashed line). An identical sample was kept in the dark as a control (solid line). The experiment shows that light causes de novo formation of a high-molecular-mass fraction.