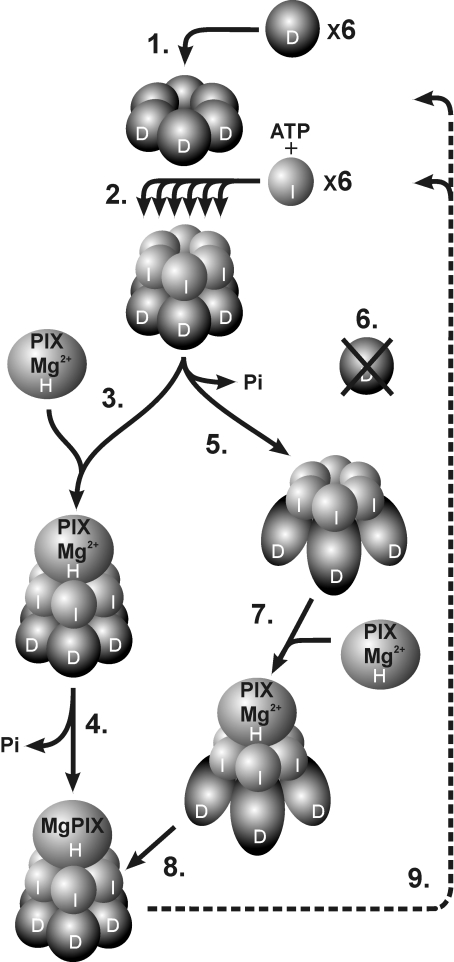
Figure 7. Model suggesting progressive subunit assembly of magnesium chelatase in relation to ATP hydrolysis.
(1) Six D-subunits form a stable hexameric ring in an ATP-independent process. (2) Using the hexameric D-ring structure as a platform, six I-subunits are assembled stepwise into a hexameric ring. The formation of the two-tiered complex is nucleotide-dependent. (3) Two alternative models are shown, which differ with respect to the timing of ATP hydrolysis. In one model the catalytic H-subunit binds to an ID complex where the ATP hydrolytic capacity of the I-subunit is blocked by the D-subunit. (4) The transient interaction with the H-subunit triggers ATP hydrolysis and insertion of Mg2+ into protoporphyrin. (5) In the alternative model, ATP hydrolysis by the I-subunits converts the D-subunits into a stable form and possibly exposes key residues required for the recognition of H-subunits. (6) Without the ATP-dependent activity of the I-subunits the D-subunits are degraded. (7) The catalytic H-subunit interacts with the activated ID complex and (8) Mg2+ is inserted into protoporphyrin without ATP hydrolysis at this step. (9) After catalysis, the IDH complex disassociates to the level of the D-hexamer, which remains for a new round of catalysis. At present, it is unknown whether the H-subunit docks to the I- or D-side of the complex, although docking to the I-subunits is shown in the Figure. PIX, protoporphyrin.