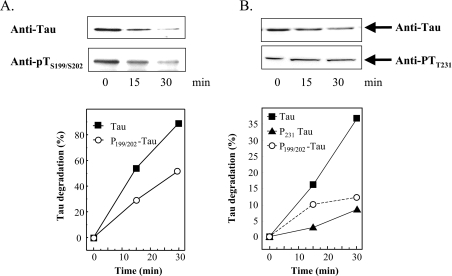
Figure 2. Tau phosphorylation inhibits tau degradation by the proteasome.
(A) Human tau was expressed in E. coli in a pET19b-plasmid (Invitrogen). Tau was purified as described by Lee et al. [37] and Keck et al. [15]. GSK3β was purchased from Biomol. Tau was phosphorylated according to Leclerc et al. [38] by adding GSK3β to purified tau (1:450 w/w) in the presence of 1 mM ATP, and incubating overnight at 30 °C. Control tau was also incubated overnight, but without GSK3β. Both non-phosphorylated tau and phospho-tau were incubated for up to 30 min with purified red blood cell 20 S proteasome (180:1, w/w) in vitro as described previously [39]. The 20 S proteasome was also purified as previously described [39]. Immunoblots, using anti-tau or anti-phospho-tau (Anti-pTS199/S202) antibodies, were performed (example shown at the top of A) and quantified (bottom of A) as described in the Materials and methods section. (B) HT22 cells were incubated for 3 h with 0.5 μM OA. The cells were then lysed and incubated with purified 20 S proteasome (180:1, w/w). The levels of two phosphorylated forms of tau, pTS199/202 and pTT231, were significantly increased by OA treatment; see Figure 3 for full details. Although tau was rapidly degraded, both pTS199/S202 and pTT231, were much more resistant to degradation by proteasome [note that the y-axis of (B) has a much smaller scale than that of (A)]. Values are means for five experiments for which S.E.M. were always less than 5%.