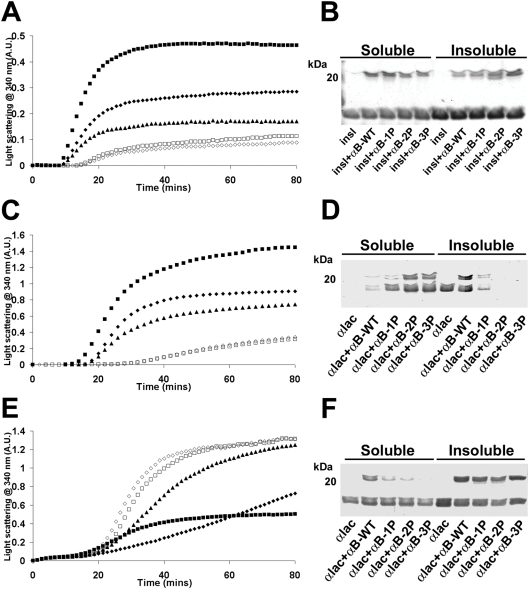
Figure 8. Effect of mimicking phosphorylation of αB-crystallin on its chaperone ability to prevent DTT-induced amorphous aggregation of target proteins.
Aggregation curves of (A) insulin (250 μg/ml) and (C, E) α-lactalbumin (500 μg/ml) incubated in the absence (■) or presence of αB-WT (◆), αB-1P (▲), αB-2P (◇) or αB-3P (□). (A) Insulin was incubated at 37 °C in 50 mM phosphate buffer (pH 7.2) with 10 mM DTT. α-Lactalbumin was incubated at 37 °C in (C) 50 mM phosphate buffer (pH 7.2) containing 100 mM NaCl with 20 mM DTT, or (E) 100 mM ammonium acetate buffer (pH 6.8) with 20 mM DTT. In each case, the chaperone (250 μg/ml) was added to the reaction mixture and the change in light scattering was measured at 340 nm. (B, D, F) After the aggregation assay, the soluble and insoluble fractions were separated and the proteins were resolved by SDS/PAGE. Each experiment was performed a minimum of three times and the results shown are representative.