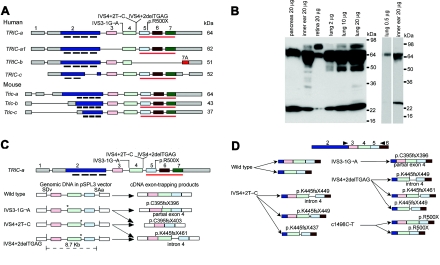
Figure 3. .
Wild-type isoforms of tricellulin, western-blot analysis, and aberrant RNA splice products that are due to mutant alleles of TRIC. A, Wild-type alternative splice isoforms of human TRIC and mouse Tric. Black bars under exon 2 show the position of the four predicted transmembrane helices. The red underline (also in panel C) indicates the occludin-ELL domain. The gray rectangles of exons 1 and 2 and of exon 7 are 5′ and 3′ UTRs, respectively. B, Western-blot analyses—through use of anti-tricellulin antibody HL5573 and protein extracts from adult C57BL/6J mouse pancreas, inner ear, retina, and lung—show a 64-kDa band in all lanes corresponding to a protein of the predicted size for Tric-a. In the retina, there is an abundant isoform of TRIC that is ∼80 kDa, which is also present at a lower concentration in lung and inner ear. In protein from lung, there is a 64-kDa band, whereas there are two bands at ∼64 kDa in protein from the cochlea. The right panel shows a western-blot analysis with a reduced amount of protein loaded on the gel for lung and a shorter exposure for the lane with protein from the cochlea. C, Aberrant splice products from exon-trapping assays. IVS3-1G→A causes the use of a cryptic splice-acceptor site within exon 4 of TRIC, which results in the deletion of the first 17 nt of exon 4 and a nonsense mutation (p.C395fsX396). IVS3-1G→A and IVS4+2T→C cause skipping of exon 4, which results in a frameshift and premature termination of translation (p.C395fsX403). IVS4+2delTGAG mRNA uses a cryptic splice-donor site in intron 4 that introduces 23 bp of intron 4, which results in a premature stop codon (p.K445fsX461). Locations of the mutant alleles are indicated by an asterisk (*). SDv and SAv are the splice-donor and splice-acceptor sites, respectively, encoded in vector sequence of pSPL3. D, Summary of RT-PCR data from lymphoblastoid cell lines of normal and (DFNB49) deaf individuals who have splice-site mutations of TRIC. The wild-type control showed the predicted splicing of exon 4 to exon 5 and alternative splicing of exon 3. mRNA isolated from lymphoblastoid cells from one deaf subject (each) of families PKDF399, PKDF443, PKDF058, and PKDF340 yielded aberrant splicing, and the locations of premature stop codons are indicated. Arrowheads indicate the locations of RT-PCR primers.