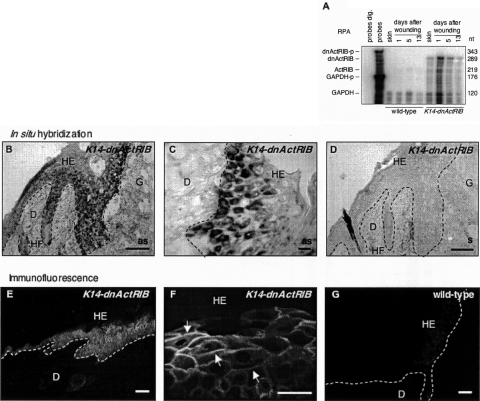
Figure 5.
dnActRIB is highly expressed in full-thickness skin wounds. A: Twenty-μg samples of total cellular RNA from normal and wounded skin of different stages from wild-type mice and transgenic animals (line Tg-868) were analyzed for the presence of dnActRIB mRNA by RPA. Hybridization with a GAPDH riboprobe served as a loading control. A low amount of the riboprobes was loaded in the lane labeled “probes” and used as a size marker. Riboprobes digested with RNases A and T1 were loaded in the lane labeled “probes dig.” B–D: Frozen sections from day 5 wounds of K14-dnActRIB transgenic mice were analyzed by nonradioactive in situ hybridization for the presence of dnActRIB mRNA. An overview over one half of a wound is shown in B. Note the strong expression of the transgene in basal and suprabasal layers of the hyperproliferative epithelium. A higher magnification of an independent section is shown in C. D: A section adjacent to the one shown in B was hybridized with the sense riboprobe. E–G: Cryosections of day 5 wounds from K14-dnActRIB transgenic (E, F) and wild-type (G) mice were analyzed by immunofluorescence using an antibody directed against the c-Myc epitope. A strong signal was detected in basal and suprabasal keratinocytes of the wound epidermis of K14-dnActRIB transgenic mice. A higher magnification (F, arrows) revealed membrane-associated localization of dnActRIB. The weak staining observed in wound epidermis of wild-type mice most likely results from the endogenous c-Myc protein. as, anti-sense; D, dermis; HE, hyperproliferative wound epidermis; HF, hair follicle; G, granulation tissue; s, sense. Scale bars: 100 μm (B, D, E, G); 50 μm (C, F).