Abstract
Both innate and adaptive immunity contribute to the progression of inflammatory-fibrotic lesions of atherosclerosis. Although platelet-derived growth factor (PDGF)-B has been investigated as a stimulant of smooth muscle cells in vascular diseases, its effects on the immune response during disease have not been evaluated in vivo. We used hematopoietic chimeras generated after lethal irradiation of ApoE−/− recipients to test the role of PDGF in atherosclerosis. Monocyte accumulation in early atherosclerotic lesions increased 1.9-fold in ApoE−/−/PDGF-B−/− chimeras. Lymphocytes from null chimeras showed a 1.6- to 2.0-fold increase in the number of activated CD4+ T cells and a 2.5-fold elevation of interferon-γ-secreting CD4+ T cells on ex vivo challenge with modified low-density lipoprotein. Splenocyte transcript levels were also altered with a twofold decrease in interleukin-10 and 1.7- and 3.0-fold increases in interleukin-18 and CCR5, respectively. These cellular and molecular changes were consistent with a shift to a proinflammatory phenotype in null chimeras. Our data also demonstrated for the first time the presence of a recently discovered family of negative regulators of innate and adaptive immunity, the suppressors of cytokine signaling (SOCS), in developing atherosclerotic lesions. Thus, our studies identify two independent negative immune regulatory pathways—PDGF-B and SOCS—that may help limit lesion expansion.
Platelet-derived growth factor (PDGF) is a pleiotropic growth factor for many cells of mesenchymal and neuroectodermal origin.1 Of the five possible isoforms of PDGF (AA, AB, BB, CC, DD) that bind to three structurally related protein tyrosine kinase type III receptors, PDGF receptor-αα, PDGF receptor-αβ, and PDGF receptor-ββ,2,3 only PDGF-BB is able to bind to all three PDGF receptors and therefore is the universal ligand. Targeted disruption of PDGF-B or PDGF receptor-β genes in mice is embryonic lethal, and mutants die at late gestation from widespread microvascular bleeding.4,5 Detailed analyses of PDGF-B- and PDGF receptor-β-null mutants have shown that this microvascular pathology is caused by a severe shortage of pericytes and vascular smooth muscle cells.6–9 Endothelial and hematopoietic cell ablations of PDGF-B have generated viable mice, and have confirmed a role for PDGF-B in regulating pericyte density and smooth muscle cell emigration in adult vascular pathologies.10–12 However, cellular effects beyond mesenchymal and neuroectodermally derived cells have not been investigated in detail in these mice.
Circulating cells, especially monocytes and platelets, are a major source of PDGF after activation or injury.13–15 Evaluation of purified populations of lymphocytes have demonstrated that PDGF can inhibit natural killer cell cytotoxicity when added to a mixed lymphocyte population16 and regulate the pattern of T-cell lymphokines produced in vitro.17 Although cultured monocyte-derived macrophages have been shown to increase expression of PDGF receptor-β with differentiation, bind PDGF-BB with high affinity, and induce PDGF receptor-β phosphorylation,18 the ability of freshly isolated monocytes to respond to PDGF has been controversial.14 Thus, PDGF has been documented to modulate activities of cultured lymphocytes and macrophages, but the implications of these in vitro effects have not been analyzed in vivo.
Both innate and adaptive immune mechanisms have been suggested to play an important role in atherosclerosis.19–22 Early lesions of atherosclerosis are composed of lipid-filled macrophages with some T cells. As lesions progress, smooth muscle cells migrate into the intima with continued accumulation of activated T cells and macrophages.15,23 The importance of innate immunity in atherogenesis is highlighted by studies in which blockade of macrophage accumulation in animal models of atherosclerosis is sufficient to delay lesion formation. For example, inhibition of either monocyte chemotactic protein-1 (MCP-1) or its receptor CCR2, known to promote monocyte migration into forming lesions, markedly decreases early lesion formation.24,25 The significance of adaptive immunity in the pathogenesis of atherosclerosis has been more controversial. A proinflammatory, Th1-type cellular immune response has been documented in human lesions,26 and multiple antigens, such as oxidatively modified lipoproteins,27 have been shown to be recognized by activated T cells isolated from lesions. Some of the differences in the interpretation of the contributions of adaptive immunity in mouse models of atherosclerosis may be due to the fact that only moderate hypercholesterolemia recapitulates the Th1 response of human disease.28,29 However, recent data in mouse models clearly demonstrate that treatments that specifically promote the Th1 response, such as blockade of transforming growth factor-β signaling specifically in T cells,30 increase lesion size and the activation state of T cells and monocytes. In contrast, B cells appear to have a protective function because removal of B cells via splenectomy enhances lesion formation, whereas intravenous polyclonal immunoglobulin injection suppresses it.31,32 How equilibrium between pro- and anti-atherogenic immune responses is balanced during disease progression is still unclear.
To test the role of PDGF-B in the development of lesions of atherosclerosis, we have examined ApoE-null mice that lack PDGF-B in their circulating cells, and our published study has demonstrated a transient delay in smooth muscle cell accumulation in advanced lesions of atherosclerosis.12 This study uses the same animal model, with a focus on the early inflammatory response and PDGF regulation of lymphocyte functions throughout lesion formation. We show that an increased accumulation of monocytes at early stages of lesion development is associated with elevated numbers of activated T lymphocytes in ApoE−/−/PDGF-B−/− chimeras that persists throughout lesion development. Analysis of lymphocyte gene expression profiles further suggests a shift to a proinflammatory phenotype and provides in vivo data implicating PDGF-B in possible modulation of T-cell activation and immune homeostasis.
Materials and Methods
Antibodies and Reagents
Conjugated rat anti-mouse antibodies (BD PharMingen, San Diego, CA) used for fluorescence-activated cell sorting (FACS) analysis were: phycoerythrin (PE) and fluorescein isothiocyanate (FITC)-B220 (RA3-6B2), PE-CD3 (17A2), PE-CD8 (53-6.7), FITC-CD4 (RM4-5), PE-Mac-1 (M1/70), biotin-CD43 (S7), biotin-Ly-5.1 (6C3/BP-1), biotin-IgM (R6-60.2), PE-CD25 [interleukin (IL)-2 receptor α chain, 3C7], PE-CD62L (l-selectin, MEL-14). Antibodies used for immunostaining include: macrophage-specific rat anti-mouse monoclonal Mac-2 antibody (diluted monoclonal supernatant33; American Type Culture Collection, Manassas, VA); suppressors of cytokine signaling (SOCS)-1-specific rabbit polyclonal antibody from Zymed (34-3100; South San Francisco, CA), SOCS-3-specific goat polyclonal antibody (m-20) from Santa Cruz Biotechnology (Santa Cruz, CA); goat anti-mouse IL-15 polyclonal antibody (AF447; R&D Systems, Minneapolis, MN); rabbit anti-human CD3 polyclonal antibody (A0452; DAKO, Carpinteria, CA).
Animals
Male ApoE−/− mice (backcrossed 10 times to C57BL/6J, G10) were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were housed in a specific pathogen-free facility and fed a normal chow diet throughout the experimental time period. First generation hematopoietic chimeras for PDGF-B were generated by total body irradiation of ApoE−/− mice 7 to 8 weeks of age and repopulation with fetal liver cells from ApoE−/−/PDGF-B +/+ or −/− E16.5 livers from littermate embryos.12 All animals used in this study were second or third generation chimeras repopulated with bone marrow from first or second generation chimeras, respectively. Venous blood was obtained by retro-orbital bleed. At the time of euthanasia, blood was drawn from the heart, and the spleen, femur, and tibia were removed. Subsequently, the aortic tree was perfused with 10 ml of phosphate-buffered saline (PBS), followed by 10 ml of methyl Carnoy’s solution (60% methanol, 30% chloroform, 10% acetic acid) and the entire aortic tree was dissected for analysis.12
Cell Preparations
No significant difference was observed in the yield of leukocytes, lymphocytes, monocytes, or macrophages between the two study groups. Leukocytes from peripheral blood, spleen, or bone marrow were prepared by ammonium chloride lysis (0.15 mol/L NH4Cl, 1.0 mmol/L KHCO3, 0.1 mmol/L ethylenediamine tetraacetic acid, pH 7.2 to 7.4) of the red blood cells, and subsequent washes with FACS buffer (PBS with 0.2% bovine serum albumin and 0.1% sodium azide) for analysis by flow cytometry. Bone marrow-derived macrophages (BMDMs) were generated by culturing bone marrow cells in RPMI 1640 containing 10% fetal bovine serum and 2000 U/ml rhM-CSF (R&D Systems) for 7 days. The BMDMs used for analysis were greater than 99% Mac-1-positive by FACS analysis. Peritoneal macrophages were collected on day 4 after intraperitoneal injection of 1 ml of aged, sterile 3% thioglycollate (Difco Laboratories, Becton Dickinson Microbiology, Sparks, MD), and further purified by adherance to bacterial plastic culture dishes for 1 hour followed by a wash to remove nonadherent cells. The resultant cell populations were greater than 99% Mac-1-positive by FACS analysis. B cells and T cells were purified from total splenocytes by negative selection to prevent adhesion-induced activation with magnetic cell sorting (MACS) beads according to the manufacturer’s protocol (Miltenyi Biotech, Bergisch Gladbach, Germany). The purity of the isolated populations was confirmed by FACS analysis after staining with a panel of cell-type-specific antibodies that included: anti-CD3, anti-B220, and anti-Mac-1. Two separate incubations with MACS beads were required to remove all B cells from T-cell and macrophage preparations, and the resultant cell populations were greater than 99% pure by FACS analysis with no detectable contaminating cells. Mouse aortic cells (primarily smooth muscle cells) were isolated from newborn mouse aorta as previously described,34 and used as a positive control for reverse transcriptase-polymerase chain reaction (RT-PCR). Splenocytes and BMDMs were incubated in serum-free media for 0.5 hour to 24 hours in the presence or absence of 30 ng/ml of recombinant PDGF-BB (endotoxin-free preparation; Roche, Indianapolis, IN). Recombinant murine IL-2 (50 U/ml, BD Pharmingen) and interferon (IFN)-γ (250 U/ml; Genzyme, Cambridge, MA) were used as positive controls for induction of SOCS proteins in splenocytes and BMDMs, respectively.
Total RNA Preparation and RNase Protection Assay
Total RNA was purified from each cell population by TRIzol reagent (InVitrogen, Carlsbad, CA) and treated with DNase to remove any contaminating DNA. Mouse chemokine, chemokine receptors, and SOCS-1 and -3 multiprobe template sets were purchased from BD PharMingen. RNA probes were synthesized with α-32P-UTP (Amersham Biosciences, Corp., Piscataway, NJ) and used within 2 days. RNase protection assays were performed according to the manufacturer’s instructions. Ten to twenty μg of total RNA was used in each hybridization reaction, and RNase-protected probe fragments were resolved with 0.4-mm 5% acrylamide gels containing 8 mol/L urea. After drying, the gels were quantitated by Phosphor Image analysis using L32 and GAPDH for normalization.
Specific Primer Sets for RT-PCR Analysis
The One-Step RT-PCR kit was purchased from Qiagen (Valencia, CA) and analysis followed the manufacturer’s protocol. Reverse transcription was performed at 50°C for 30 minutes, then 95°C for 15 minutes to heat-inactivate the reverse transcriptase. PCR conditions are: 94°C, 1 minute; 55°C, 1 minute; and 72°C, 1 minute. Thirty to thirty-five cycles were used, depending on the abundance of the message. Specific murine primer sets are shown in Table 1.
Table 1.
Specific Murine Primer Sets Used in This Study
| Genes | Sequence | Expected size |
|---|---|---|
| PDGF-Rα | Sense: 5′-AAGAGACCCTCCTTCTACCACC-3′ | 399 bp |
| Anti-sense: 5′-GTCGATGTCCTCGATGGTCT-3′ | ||
| PDGF-Rβ | Sense: 5′-AGCTACATGGCCCCTTATGA-3′ | 367 bp |
| Anti-sense: 5′-GGATCCCAAAAGACCAGACA-3′ | ||
| PDGF-A | Sense: 5′-CCATTCGCAGGAAGAGAAGTA-3′ | 436 bp |
| Anti-sense: 5′-GGCAATGAAGCACCATACATA-3′ | ||
| PDGF-B | Sense: 5′-CGACCACTCCATCCGCTCCTT-3′ | 461 bp |
| Anti-sense: 5′-GCCGAGGGGTCACTATTGTCT-3′ | ||
| PDGF-C | Sense: 5′-GAGTCCAACCTGAGCAGCAAG-3′ | 519 bp |
| Anti-sense: 5′-GGTACTGAAGGCAGTCACAGC-3′ | ||
| PDGF-D | Sense: 5′-CTTCCCGAACAGCTACCCAAG-3′ | 559 bp |
| Anti-sense: 5′-CCGATCATGGTATGACCTGCC-3′ | ||
| L32 | Sense: 5′-GCCGCTTTCTGCTTAGCTTG-3′ | 520 bp |
| Anti-sense: 5′-CAGAGTGTCTTCCAATCACC-3′ | ||
| GAPDH | Sense: 5′-GACTCCACTCACGGCAAATTC-3′ | 575 bp |
| Anti-sense: 5′-GACACATTGGGGGTAGGAACA-3′ |
Cell Staining and Flow Cytometry Analysis
Cells were resuspended at 2 to 3 × 106/ml, and 200 μl of cell suspension were incubated in a 96-well plate with specific antibodies for 20 minutes at room temperature in the dark. Fcγ antibody was added before staining with specific antibodies to block nonspecific binding. Stained cells were washed with the FACS buffer (PBS with 0.2% bovine serum albumin and 0.1% NaN3) three times before analysis on FACScan flow cytometer (Becton Dickinson).
IFN-γ Secretion and Detection Assay
Specific IFN-γ secretion on ex vivo challenge with malondialdehyde (MDA)-low density lipoprotein (LDL) was evaluated using a cell enrichment and detection kit (Miltenyi Biotech). Fresh mouse spleen cells were prepared under sterile conditions. Ten million splenocytes were incubated at 37°C for 3 to 16 hours in 1 ml of RPMI 1640/5% bovine plasma-derived serum in the presence of 10 μg/ml MDA-LDL. MDA modification of LDL was performed as described in detail previously.35 Plasma-derived serum was used in the place of serum to minimize exposure of the cells to PDGF present in serum but absent in plasma. A negative control (with no antigen stimulation) and a positive control (stimulated by 10 μg/ml of Staphylococcal Enterotoxin B; Sigma, St. Louis, MO) were included in each assay. IFN-γ-secreting cells were labeled by IFN-γ catch reagent (CD45 antibody conjugated to anti-IFN-γ), and then labeled with PE-anti-IFN-γ detection antibody. Because the frequency of IFN-γ-secreting cells is less than 2%, positive cells were then labeled with anti-PE MicroBeads and magnetically enriched. The enriched population was also stained with FITC-CD4 antibody and analyzed by FACScan flow cytometry to evaluate CD4+/IFN-γ secreting cells, which were specific to MDA-LDL.
Immunohistochemistry and Quantitation
All immunohistochemical procedures were performed as previously described.35 Monocytes in brachiocephalic artery of ApoE−/−/PDGF-B chimeras from 23 to 26 weeks of age were evaluated by immunostaining with Mac-2 antibody.33 Using a random start site, the entire brachiocephalic trunk was sampled at 75-μm intervals as previously described.12 SOCS-1 and SOCS-3 expression were evaluated by staining brachiocephalic sections with SOCS-1- and SOCS-3-specific antibodies (both at 2 μg/ml). Adjacent sections were stained with Mac-2 antibody to evaluate co-localization of SOCS proteins and macrophages. Lesion T cells and IL-15-positive cells were evaluated by staining longitudinal sections of the thoracic aorta and its major branches (excluding the brachiocephalic artery) with anti-CD3 antibody (5 μg/ml) and anti-IL-15 antibody (5 μg/ml). All analyses and counting were done blinded to the tissue source, and the number of positive cells per section were counted by two independent observers. NIH Image software was used to quantify the lesion area (mm2) and vessel length (cm).
Statistics
Statistical analysis was performed using a two-tailed Student’s t-test and the InStat 2.01 program.
Results
Enhanced Monocyte Accumulation at Early Stages of Atherosclerotic Lesion Formation in ApoE−/−/PDGF-B−/− Chimeras
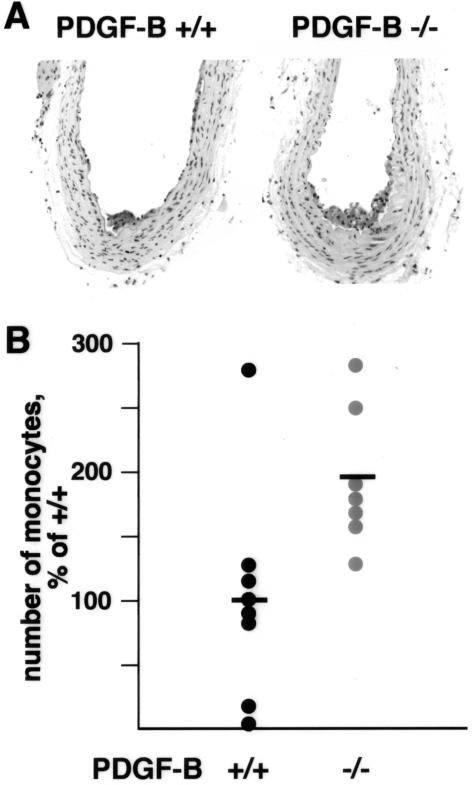
Because targeted deletion of the PDGF-B chain is embryonic lethal, we have developed hematopoietic chimeras using fetal liver cells from ApoE−/−/PDGF-B-deficient embryos to repopulate the marrow of lethally irradiated atherosclerosis-prone ApoE−/− mice. Using this model, our previous study has shown that at 35 weeks of age (28 weeks after transplantation), lesions in PDGF-B−/− chimeras contain mostly macrophages, appear less mature, and have a reduced frequency of fibrous cap formation as compared with PDGF-B+/+ chimeras.12 To further examine the inflammatory response in these chimeras, we evaluated macrophage content and the nature of the lesions at an earlier stage, 23 to 26 weeks of age (16 to 19 weeks after transplantation) (Figure 1A). Because these lesions contain only macrophages, lesions were quantitatively compared by determining the number of intimal monocytes/macrophages in the entire brachiocephalic trunk in mice at 23 to 26 weeks of age. Our data demonstrates a 1.9-fold increase in the number of Mac-2-positive cells accumulated in lesions of ApoE−/−/PDGF-B−/− chimeras (Figure 1B), reflecting the apparent increase in lesion size at this stage of development.
Figure 1.
Increased number of monocytes in atherosclerotic lesions of 23- to 26-week ApoE−/−/PDGF-B−/− chimeras. Hematopoietic chimeras were generated by lethal irradiation of 7-week-old ApoE−/− mice and repopulation of their marrow with ApoE−/−/PDGF-B+/+ or ApoE−/−/PDGF-B−/− bone marrow cells from first generation chimeras.12 Mice were fed a normal chow diet and euthanized at 23, 24, and 26 weeks of age (23 weeks, n = 2 for each group; 24 weeks, n = 4 for +/+ and n = 3 for −/−; and 26 weeks, n = 2 for each group). After perfusion fixation with methyl Carnoy’s and paraffin embedding, the entire brachiocephalic trunk was serially sectioned. A: Immunostaining with monocyte/macrophage-specific Mac-2 antibody was used to quantitate lesion monocytes in PDGF-BB+/+ and PDGF-BB−/− chimeras. At this stage of lesion development, monocytes account for all of the intimal lesion area. B: The number of lesion monocytes were quantitated at 75-μ intervals for the full length of the brachiocephalic trunk for PDGF-BB+/+ (n = 8) and PDGF-BB−/− (n = 7) chimeras. Because mice were sacrificed at different time points and were part of three different experimental groups, each data set was analyzed separately and expressed as the percentage of the mean for the PDGF-BB+/+ chimeras in that group.
PDGF-B Transcript Is the Major PDGF Ligand Gene Detected in All Mouse Hematopoietic Lineages, but Receptor Expression Is More Limited
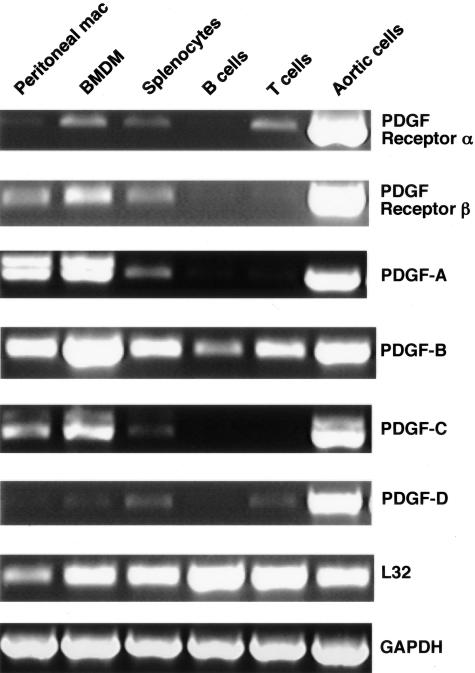
Although monocytes/macrophages are known to produce PDGF-B through the analysis of atherosclerotic lesions from humans, nonhuman primates,13,36 and mice,12 expression of other PDGF ligands and receptors have not been well characterized in mouse hematopoietic cells. RT-PCR analyses were used to examine mRNA expression for PDGF ligands and receptors in wild-type C57BL/6 mice (Figure 2). Mouse aortic cells (primarily smooth muscle cells) were used as a positive control. PDGF-B mRNA is expressed in all hematopoietic lineages tested, whereas PDGF-A and PDGF-C are limited to macrophages and total splenocytes, presumably from nonlymphatic cells, because no expression is observed in purified B and T cells. Expression of PDGF-D in circulating cells is minimal in comparison with the other ligands.
Figure 2.
Differential expression of PDGF receptors and ligands in mouse hematopoietic lineages. Thioglycollate-elicited peritoneal macrophages, BMDMs, and splenocytes were prepared from wild-type male C57BL/6J mice. B cells and T cells were purified from total splenocytes by negative selection as described in Materials and Methods. Mouse aortic cells (primarily smooth muscle) were isolated from newborn mouse aorta and used as a positive control. Total RNA was purified from each cell population and treated with DNase to remove contaminating DNA. Two hundred ng of purified RNA was used in a One-Step RT-PCR reaction with specific primer pairs as indicated in Table 1 and the PCR products were run on a 1% agarose gel using L32 and GAPDH as controls for RNA load. Comparable expression levels were observed in hematopoietic cells from ApoE−/− mice (data not shown).
PDGF receptor-α mRNA is detected in BMDMs, splenocytes, and purified T cells. Neither purified B nor T cells express PDGF receptor-β mRNA, but it is detected in thioglycollate-elicited peritoneal macrophages, BMDMs, and splenocytes. This suggests that naïve T cells are dependent on PDGF receptor-α to respond to PDGF, and thus can be stimulated by PDGF-AA, -AB, -BB, and -CC. Peritoneal macrophages and BMDMs differ in their PDGF receptor expression. Although BMDMs have both receptors-α and -β, peritoneal macrophages express only receptor-β. A similar pattern of PDGF ligand and receptor expression is observed in hematopoietic cells from hypercholesterolemic ApoE−/− mice (data not shown).
The Number of Activated T Lymphocytes Is Increased in ApoE−/−/PDGF-B−/− Chimeras
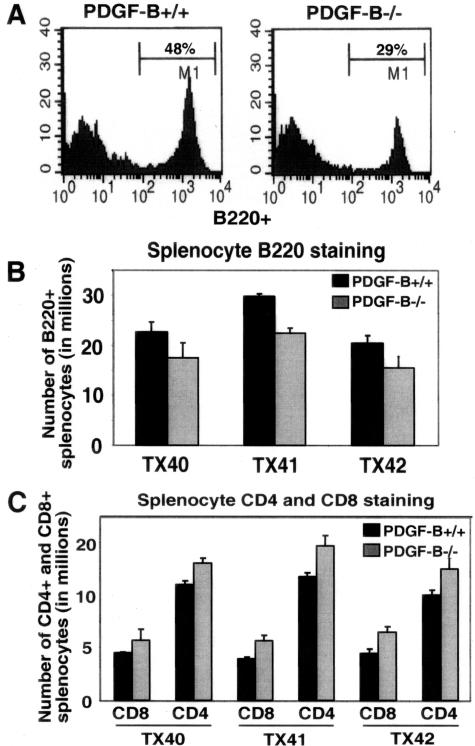
Given the broad expression of PDGF-B in all circulating cells, we asked whether hematopoietic cell distribution is altered in its absence. As previously reported, no difference in the total numbers of lymphocytes, granulocytes, or macrophages is observed in PDGF-null chimeras,12 but individual lymphocyte populations have not been examined. Using flow cytometric analysis, a significant 30 ± 8% decrease in the number of B220+ cells and a 30 ± 7% increase in CD4+ and CD8+ cells is observed in the spleen (Figure 3) and peripheral blood (not shown) of ApoE−/−/PDGF-B−/− chimeras as compared with PDGF-B+/+ chimeras, whereas the total number of splenocytes and circulating leukocytes are the same between the two groups. The changes in both B- and T-cell numbers are statistically significant, and are consistent from 14 to 45 weeks of age (data not shown). The reduction in B220+ cells does not appear to be due to a block in B-cell development, because flow cytometric analysis of bone marrow cells for progenitor B cells (B220/CD43), pre-B cells (B220/BP-1), and IgM-bearing B cells (B220/IgM) revealed no blockage of B-cell differentiation in PDGF-B−/− chimeras (data not shown). The increase in T-cell numbers is also unlikely to be due to defects in T-cell development because the ratio of CD8 to CD4 is not altered in these chimeras.
Figure 3.
B-lymphocyte numbers are decreased and T-lymphocyte numbers are increased in the periphery of ApoE−/−/PDGF-B−/− chimeras. A: Representative FACS analyses are shown of B220 staining of splenocytes from 30-week-old PDGF-B+/+ and −/− chimeras gated for lymphocytes. B: B-cell numbers are decreased in spleens of ApoE−/−/PDGF-B−/− chimeras, although total lymphocyte numbers are comparable for the two chimera groups. Flow analysis of B220 staining of three independent transplant (TX) experiments at 30 weeks are shown (n = 3 per group, P < 0.05). C: The decrease in B cells is associated with an increase in T cells in ApoE−/−/PDGF-B−/− chimeras (n = 3 per group, P < 0.01). CD4 and CD8 staining was performed with the same splenocyte samples as in B.
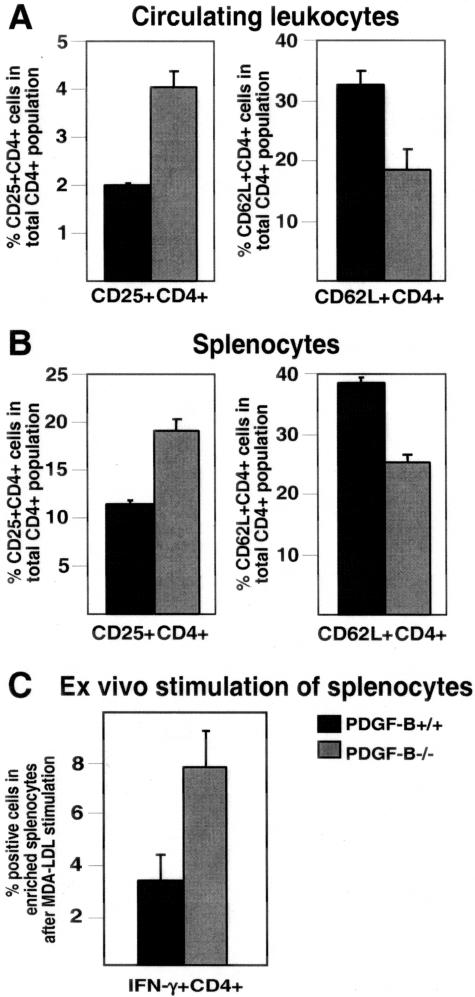
These studies show an increase in the number of T cells in PDGF-B-null chimeras (Figure 3), and we previously demonstrated that these mice have increased macrophage expression of IFN-γ, a potent modulator of T-cell activation.12 Therefore, we tested whether T-cell activation patterns differ between the PDGF-B+/+ and −/− chimeras. A transient increase in cell surface expression of CD25 is associated with T-cell activation, whereas CD62L (l-selectin) is shed from the cell surface by activated T cells. PDGF-B−/− chimeras show a twofold ± 0.2 increase in the CD25/CD4 double-positive cell population, and a 1.8-fold ± 0.1 decrease in the CD62L/CD4 double-positive cell population in their peripheral blood leukocytes and spleens (Figure 4, A and B). Even though 10 to 20% of CD25/CD4 double-positive cells are regulatory T cells,37 the majority of these double-positive cells are activated T cells. The combined data for these markers of T-cell activation show that the number and percentage of activated T cells is increased in ApoE-null mice lacking PDGF-B in their circulating cells.
Figure 4.
Increased numbers of activated T lymphocytes in ApoE−/−/PDGF-B−/− as compared to PDGF-B+/+ chimeras. A: From PDGF-B+/+ (black bars) and PDGF-B−/− (gray bars) chimeric mice, peripheral blood leukocytes were stained with antibodies to CD25 or CD62 ligand in combination with a CD4 antibody and double-positive cell populations were analyzed by flow cytometry (n = 6 per group, P = 0.004 for CD25+CD4+, and P = 0.008 for CD62L+CD4+). B: Splenocytes from the same mice were also stained and analyzed as described in A (P < 0.01). C: Freshly isolated splenocytes from ApoE−/−/PDGF-B+/+ and −/− chimeras were incubated with 10 μg/ml of MDA-LDL overnight. IFN-γ secreting and CD4+ cells were labeled, enriched using magnetic beads, and analyzed by flow cytometry (see Materials and Methods). An increase in the IFN-γ+CD4+ population is detected in ApoE−/−/PDGF-B−/− chimeras (n = 6 for +/+ chimera, n = 5 for −/− chimera; P = 0.0009).
Modified lipoproteins have been implicated as immune stimuli in atherogenesis,38,39 including the promotion of T-lymphocyte activation within lesions.39 To test the extent to which T cells from PDGF-B−/− chimeras are activated by atherogenic stimuli, freshly isolated splenocytes from ApoE−/−/PDGF-B+/+ and −/− chimeras were incubated ex vivo with malondialdehyde-modified LDL (MDA-LDL). Overnight incubation with MDA-LDL increased the number of IFN-γ secreting CD4+ T cells in PDGF-B−/− chimeras by 2.5-fold ± 0.4 as compared with PDGF-B+/+ chimeras (Figure 4C). These data demonstrate that T cells from PDGF-B chimeras have an enhanced response to an atherogenic stimulus ex vivo.
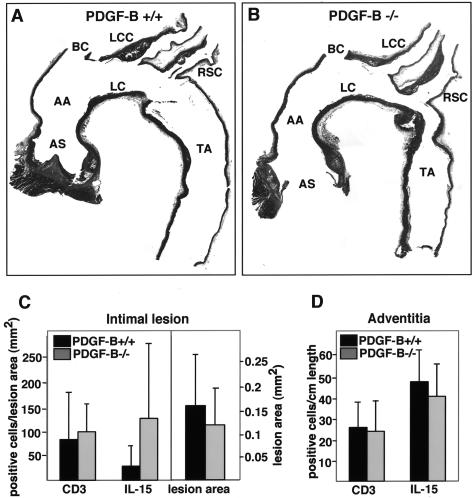
Because the number of activated T cells in the circulation is increased in PDGF-B−/− chimeras, we examined whether T-cell accumulation in lesions is altered. To optimize quantitation of lymphocytes, we evaluated longitudinal sections of the thoracic aorta and its major branches from the ascending aorta to the base of the thoracic aorta (Figure 5, A and B). CD3-positive cells were counted in both the intima and the adventitia normalizing the count to the length of vessel for the adventitial cells and lesion volume for lesional cells. The number of CD3-positive cells in the intima and adventitia of 45-week-old PDGF-B+/+ and PDGF-B−/− chimeras was comparable, as were lesion areas (Figure 5, C and D). Similar data were obtained with 35-week-old PDGF-B chimeras (data not shown). Although T-cell activation and IFN-γ expression in lesions may differ between the two experimental groups, we were unable to stain for IFN-γ in our methyl Carnoy’s-fixed specimens using three different IFN-γ antibodies.
Figure 5.
Detection of CD3- and IL-15-positive cells in longitudinal sections of ApoE−/−/PDGF-B chimeras. A, B: Representative aortic longitudinal sections stained with H&E from 45-week-old ApoE−/−/PDGF-B+/+ (A) and ApoE−/−/PDGF-B−/− (B) chimeras are shown. The location of the different vessels is shown: AS, aortic sinus; AA, ascending aorta; BC, brachiocephalic; LC, lesser curvature; LCC, left common carotid artery; RSC, right subclavian artery; TA, thoracic aorta. The brachiocephalic artery was removed where it branches off of the aorta and was embedded separately for analysis. C: Lesion T cells and IL-15-positive cells were evaluated by staining longitudinal sections of the thoracic aorta of 45-week-old chimeras (PDGF-B+/+, n = 8; PDGF-B−/−, n = 7) with anti-CD3 and anti-IL-15 antibodies, respectively. Lesion area is also shown for the two groups, and CD3- and IL-15-positive cells in intima were normalized to lesion area (mm2). D: CD3- and IL-15-positive cells in the adventitia were also counted and normalized to vessel length.
Probing Possible Mechanisms of T-Cell Activation in ApoE−/−PDGF-B−/− Chimeras
In the absence of circulating PDGF-B in ApoE−/− mice, we show increased monocyte/macrophage infiltration during early stages of lesion development (Figure 1). Our previous analysis of alterations in macrophage gene expression in PDGF-B-deficient chimeras demonstrated increased expression of IFN-γ and the cytokines IL-1α and IL-15.12 Because IL-15 promotes the activation and recruitment of T cells to sites of inflammation including atherosclerotic lesions,40,41 we also evaluated whether IL-15 expression is changed in lesions of PDGF-B−/− chimeras. No difference was seen in the number of IL-15-positive cells in the adventitia between PDGF-B+/+ and PDGF-B−/− chimeras (Figure 5D), although T-cell accumulation in the adventitia was fivefold higher than within the lesion when both were normalized to vessel length (data not shown). In contrast, the number of intimal IL-15-positive cells appears to be increased fourfold in ApoE−/−/PDGF-B−/− chimeras as compared to PDGF-B+/+ chimeras (Figure 5C). However, the difference did not quite reach statistical significance.
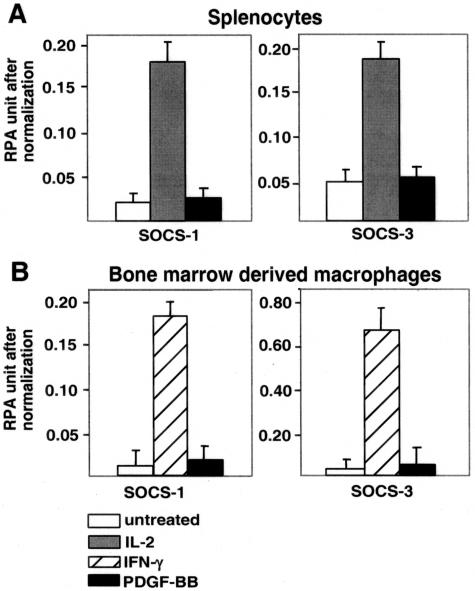
Another possible candidate to mediate the observed increase in T-cell activation in PDGF-null chimeras is a recently discovered family of negative inhibitory factors, the SOCS, that has been shown to function as negative feedback regulators of inflammatory cytokine signaling. We and others have found that PDGF alone significantly up-regulates SOCS-1 and SOCS-3, two key members of this family,42 in NIH3T3 cells that express high levels of both PDGF receptors-α and -β. However, PDGF-BB fails to stimulate SOCS-1 or SOCS-3 expression in cultured splenocytes and BMDMs (Figure 6), even though the cells express low levels of PDGF receptor-α and -β mRNA (Figure 2). In contrast, IL-2 and IFN-γ can stimulate SOCS expression in splenocytes and BMDM, respectively, confirming the responsiveness of these cultured cell populations. Potential synergistic effects of PDGF-B with other SOCS inducers were not evaluated.
Figure 6.
PDGF-BB does not increase the expression of SOCS-1 and SOCS-3 in mouse splenocytes and BMDMs in culture. Splenocytes and BMDMs were prepared from wild-type male C57BL/6J mice and incubated in serum-free media for 0.5 hours to 24 hours in the presence (black bars) or absence (white bars) of 30 ng/ml of recombinant PDGF-BB. Recombinant murine IL-2 (50 U/ml, gray bars) and IFN-γ (250 U/ml, striped bars) were used as positive controls for splenocytes and BMDMs, respectively. Total RNA (10 μg) was analyzed by RNase protection assays (RPAs) with probes specific to mouse SOCS-1 and SOCS-3. Gels were quantitated by Phosphor Image analysis using L32 and GAPDH for normalization. Similar data were obtained with cells from 24-, 35-, and 45-week-old PDGF-B chimeras. Representative data for splenocytes (A) and BMDMs (B) that were incubated for 6 hours as indicated are shown.
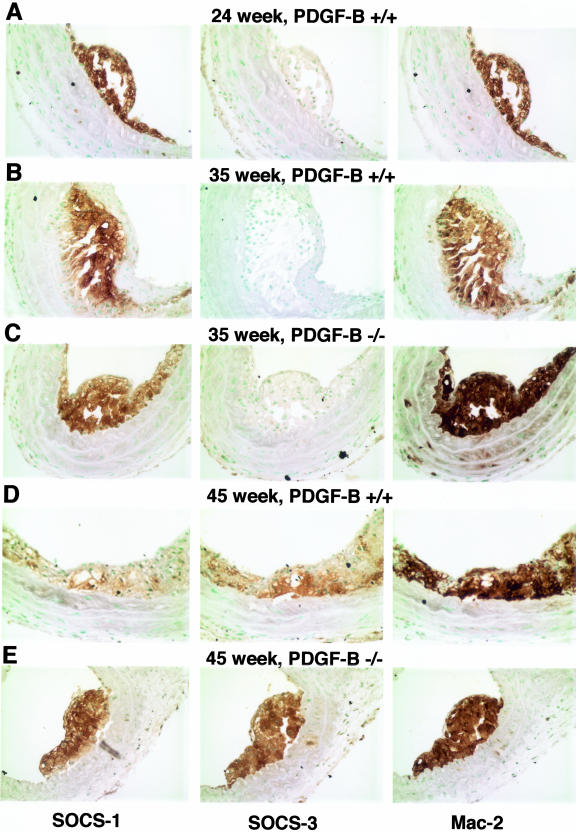
SOCS expression has not been evaluated in lesions of atherosclerosis, and it is possible that the cell culture environment does not mimic the immune stimulation within atherosclerotic lesion. Immunohistochemical analysis of lesions reveals strong staining for both SOCS-1 and SOCS-3 that co-localizes with lesion macrophages (Figure 7). SOCS-1 is expressed at all time points examined, while SOCS-3 is more evident at later stages of lesion development (greater than 40 weeks in the chimeric mice). However, no differences in SOCS-1 or SOCS-3 staining is observed in lesions of age-matched ApoE−/−/PDGF-BB+/+ and −/− animals, consistent with an absence of SOCS mRNA regulation by PDGF-B in cultured cells. However, immunohistochemical evaluation of SOCS expression would only allow detection of a difference between the two groups if PDGF-B is a major contributor to SOCS induction within lesions.
Figure 7.
Both SOCS-1 and SOCS-3 are detected in developing lesions of atherosclerosis and are co-localized with lesion monocytes/macrophages. SOCS-1- and SOCS-3-specific antibodies and monocyte/macrophage-specific Mac-2 antibody were used to stain sections from the brachiocephalic trunks of 24-week ApoE−/−/PDGF-B+/+ chimeras (A); 35-week ApoE−/−/PDGF-B+/+ (B); 35-week ApoE−/−/PDGF-B−/− (C); 45-week ApoE−/−/PDGF-B+/+ (D); and 45-week ApoE−/−/PDGF-B−/− chimeras (E).
The Gene Expression Profile in PDGF-B−/− Chimera Splenocytes Shows a Selective Shift to a Proinflammatory Phenotype
To analyze whether the gene expression is changed in hematopoietic cells in the absence of PDGF-B, total RNA was purified from spleens of 24-week-old PDGF-B chimeras and mRNA transcripts of chemokines/cytokines and their receptors were assessed by RNase protection assay. Data in Table 2 show a 2.0-fold decrease in anti-inflammatory IL-10 and a 1.7- and 3-fold increase in proinflammatory IL-18 and CCR5 expression, respectively. A more modest reduction in IL-6Rα and IL-12Rβ2 expression is observed. Evaluation of several other cytokines was precluded by low expression levels (Table 2). The observed changes in the splenocyte gene expression profile in PDGF-B−/− chimeras suggests selective enhancement of inflammatory pathways.
Table 2.
Summary of Gene Alterations in Splenocytes Evaluated by RNase Protection Assays
| Genes | Fold differences |
|---|---|
| Chemokines/cytokines | |
| IL-1α | 1.0 ± 0.2 (n = 3) |
| IL-10 | 0.5 ± 0.1 (n = 3) |
| IL-15 | 0.9 ± 0.2 (n = 3) |
| IL-18 | 1.7 ± 0.2 (n = 3) |
| Lymphotoxin | 0.7 ± 0.1 (n = 3) |
| Macrophage-inflammatory protein-1α (MIP-1α) | 1.1 ± 0.3 (n = 3) |
| MIP-1β | 1.0 ± 0.2 (n = 3) |
| MIP-2 | 1.0 ± 0.1 (n = 3) |
| RANTES | 0.9 ± 0.2 (n = 3) |
| Chemokine/cytokine receptors | |
| C-C chemokine receptor-1 (CCR1) | 1.0 ± 0.2 (n = 3) |
| CCR2 | 1.4 ± 0.5 (n = 3) |
| CCR5 | 3.0 ± 1.6 (n = 3) |
| IL-4Rα | 1.0 ± 0.1 (n = 2) |
| IL-6Rα | 0.6 ± 0.1 (n = 2) |
| IL-12Rβ2 | 0.7 ± 0.1 (n = 2) |
| IL-13Rα | 0.9 ± 0.3 (n = 2) |
| IL-15Rα | 1.1 ± 0.3 (n = 2) |
Data presents the fold differences in splenocytes isolated from 24-week-old ApoE−/−/PDGF-B−/− chimeras as compared to +/+ chimeras after normalization.
Number after ± represents SD or differences in average.
Other genes included in the multiprobe sets that gave signals too low to allow interpretation were: granulocyte macrophage colony-stimulating factor (GM-CSF), IFN-γ, MCP-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-13, CCR1B, and CCR4.
Discussion
PDGF-B as a Potential Regulator of T-Cell Activation and the Proinflammatory Response
This study shows that ApoE hematopoietic chimeras deficient in PDGF-B have a twofold increase in the number of activated T cells with a shift in the splenocyte gene profile to a selective proinflammatory phenotype, and thus implicates PDGF-B as a potential modulator of the immune response. We further demonstrate that PDGF-B mRNA is the primary PDGF transcript in all freshly isolated hematopoietic lineages, whereas transcripts for PDGF-A and -C are limited to nonlymphoid cells (primarily macrophages). Purified T cells only express transcripts for PDGF receptor-α and thus can respond to macrophage-derived PDGF-AA, -AB, -BB, and -CC or to PDGF-BB made by both T and B lymphocytes. Our combined data with freshly isolated hematopoietic cells from wild-type mice and PDGF-B hematopoietic chimeras are consistent with the previous in vitro data that PDGF-BB depresses immune-modulating cytokines43 and provide support for possible PDGF-B down-modulation of T-cell immune responsiveness in vivo.
Our initial characterization of lesion progression in the ApoE/PDGF-B-null chimeras demonstrated a twofold increase in macrophage proinflammatory gene expression, including IFN-γ, IL-1α, IL-15, and the cytokine receptor CCR5.12 In this study, we also show an increase in CCR5 expression in splenocytes of PDGF-B−/− chimeras as well as an increase in IL-18. Although T cells are considered a primary source of IFN-γ, it has more recently been shown that IL-18 in combination with IL-12 induces IFN-γ in macrophages44,45 and we observe enhanced macrophage IFN-γ expression in PDGF-B-null chimeras that have increased splenocyte expression of IL-18. Although we were unable to directly test the implications of the gene expression changes in vivo, overexpression of CCR5 has been shown to increase the migratory rate of T cells toward RANTES and MIP-1α,46 which could promote T-cell accumulation and activation within lesions. The enhanced IL-18 expression by splenocytes would also be predicted to be proinflammatory because its blockade decreases macrophage and T-cell accumulation and lesion progression in ApoE-null mice.47 Although we did not detect a difference in T-cell accumulation within lesions, we show an increase in the number of macrophages in PDGF-B-null chimeras at early stages of lesion formation. Altered macrophage gene expression could locally promote T-cell activation. IL-15 was elevated in PDGF-null macrophages,12 and it promotes T-cell activation, proliferation, recruitment, and has previously been localized to lesions of atherosclerosis.40,41 Although the data did not quite reach statistical significance, we observed a trend toward an increase in the number of IL-15-positive cells in lesions of PDGF-B-null chimeras. In addition to enhanced proinflammatory gene expression in splenocytes of ApoE−/−/PDGF-B−/− chimeras, we also observe a decrease in IL-10 expression, a suppressor of the immune response. We posit that the proinflammatory phenotype of macrophages and lymphocytes in PDGF-B-null chimeras may have contributed to lesion progression in these chimeras, including comparable lesion size and complexity at 45 weeks of age despite a delay in fibrous cap formation at 35 weeks.12 The selective proinflammatory shift in lymphocyte and macrophage phenotype in PDGF-B-null chimeras further implicates PDGF-B in immune cell homeostasis for normal hematopoietic cells.
Negative Regulators of the Inflammatory Response May Help Limit Lesion Expansion
There has been increasing recognition of the critical role played by pathways that limit potent cytokine signaling. In our search for a possible mechanism through which PDGF might normally limit proinflammatory responses, a likely possibility was the family of negative regulators—the SOCS.48 We and others have found that PDGF significantly up-regulates SOCS-1 and SOCS-3, two key members of this family,42 in NIH3T3 cells that express high levels of both PDGF receptors-α and -β. However, despite the fact that we detect PDGF receptor expression on T cells and macrophages, we fail to detect PDGF-mediated SOCS expression in isolated splenocytes or macrophages.
Although SOCS-1 and SOCS-3 do not appear to be major downstream targets of PDGF-B in lymphocytes or macrophages, we provide evidence for the first time that both SOCS-1 and SOCS-3 are present in lesions of atherosclerosis. Although SOCS-1 expression is detected throughout lesion development in ApoE chimeras (24 weeks to 50 weeks), SOCS-3 expression becomes more evident at later stages (45 weeks and older). Both SOCS-1 and SOCS-3 co-localize with Mac-2-positive lesion macrophages. SOCS proteins are an integral part of the immune response serving as a cytoplasmic-negative feedback loop to prevent excessive response to cytokines.48 In vivo, enhanced monocyte infiltration and T-cell activation was observed in a model of acute inflammatory arthritis in the absence of SOCS-1.49 It will be interesting to determine whether SOCS-1 and/or SOCS-3 expression is modulated in other models of atherosclerosis in which the immune and inflammatory response is dysregulated, such as blockade of TGF-β signaling. Because excessive inflammation is thought to tip the balance toward progression of atherogenesis, strategies that promote SOCS-1 or SOCS-3 expression may prove to be beneficial.
How Does PDGF-B Expression Modulate Immune Cell Function in Vivo?
Our in vivo studies highlight a potential role for hematopoietic cell-derived PDGF-B in immune cell homeostasis. However, they do not allow us to determine whether PDGF-B from normal hematopoietic cells acts directly or indirectly to mediate these effects. Given the potent effects of PDGF on mesenchymal cells, macrophage- and lymphocyte-derived PDGF-B may stimulate mesenchymal cells in the stroma or vessel wall that may subsequently induce secretion of factors that are responsible for modulating macrophage and lymphocyte responses, similar to what has been shown for hematopoietic precursors.50–52
Isolated T cells from mouse lymph nodes as well as T-cell hybridomas have been shown to respond directly to PDGF-BB in serum-free media by modulating their cytokine production, including down-regulation of IFN-γ.53 A later study raises the possibility that specific cytokines and tissue-specific microenvironments may regulate the sensitivity of T cells to PDGF-mediated influences.54 Responsiveness of T cells to PDGF is abrogated by IL-6 stimulation, and T cells residing in lymphoid organs that receive their major afferent lymphatic drainage from gut mucosa (in contrast to the vast majority of peripheral T cells) are much less responsive to PDGF stimulation.54 Thus, although T cells can directly respond to PDGF, this response can be modulated by soluble factors and microenvironment.
Highly purified monocytes have been shown to require addition of either lymphocytes or IL-1 to respond chemotactically to PDGF-AA.55 Similarly, we show that mouse peritoneal macrophages express PDGF receptor-β but not the PDGF receptor-α. Lymphocyte-monocyte interactions and/or cytokine stimulation may thus be required to induce PDGF receptor-α on monocytes while monocyte-derived macrophages appear to express PDGF receptor-β after differentiation18 and can therefore respond to their own PDGF-B or that from other neighboring cells.
The failure of PDGF to induce SOCS expression in splenocytes and macrophages is surprising given the known stimulation of SOCS by PDGF in mesenchymal cells.42 However, a key observation from the analysis of allelic PDGF receptor mutations may provide an explanation. In vivo, even a small reduction in the gene dosage of functional PDGF receptor had a significant impact on the recruitment of PDGF-β receptor-dependent pericytes and vascular smooth muscle cells.56 In contrast with characterized mesenchymal cells, estimates of PDGF receptor-β expression levels on monocyte-derived macrophages suggest a receptor number 10 to 20% of that observed in human skin fibroblasts.18 Thus, a reduced level of PDGF receptor expression on lymphocytes and macrophages could induce distinct signaling pathways or signals of insufficient magnitude to induce SOCS-1 and SOCS-3. We have also not investigated the possible synergistic effects of PDGF in combination with other stimulants.
Understanding the nature of PDGF signaling in lymphocytes and macrophages that appear to express much lower levels of PDGF receptors than mesenchymal cells, and determining whether PDGF acts directly and/or indirectly on macrophages and lymphocytes, will be important for better defining PDGF’s role in immune cell homeostasis. However, our studies with PDGF-B-null hematopoietic chimeras clearly show that the absence of PDGF-B in the circulating cell population is sufficient to increase macrophage accumulation at early stages of lesion formation, alter the pattern of macrophage and lymphocyte gene expression to a more proinflammatory phenotype, and these changes in gene expression are associated with an increase in the percentage of activated T cells. Thus, one of the normal functions of PDGF-B in hematopoietic cells may be to reduce the inflammatory response associated with chronic insults such as hypercholesterolemia in atherosclerosis.
Acknowledgments
We thank Roderick Browne, Kelli McIntyre, Li-Chuan Huang, and Bonnie Ashleman for expert technical assistance; and Carole Balach for editorial assistance.
Footnotes
Address reprint requests to Elaine W. Raines, Harborview Medical Center, 325 9th Ave, Box 359675, Seattle, WA 98104-2499. E-mail: ewraines@u.washington.edu.
Supported by the National Institutes of Health (grant HL18645 to E.W.R. and training grant HL07828 to J.T.), the Swedish Medical Research Council, Cancer Foundation, Inga-Britt and Anne Lundberg Foundation, and Novo Nordisk Foundation (to C.B.).
References
- Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Ohlsson R, Falck P, Hellstrom M, Lindahl P, Bostrom H, Franklin G, Ahrlund-Richter L, Pollard J, Soriano P, Betsholtz C. PDGFB regulates the development of the labyrinthine layer of the mouse fetal placenta. Dev Biol. 1999;212:124–136. doi: 10.1006/dbio.1999.9306. [DOI] [PubMed] [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes H, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski WE, Lindahl P, Lin NL, Broudy VC, Crosby JR, Hellstrom M, Swolin B, Bowen-Pope DF, Martin PJ, Ross R, Betsholtz C, Raines EW. Basis of hematopoietic defects in platelet-derived growth factor (PDGF)-B and PDGF beta-receptor null mice. Blood. 2001;97:1990–1998. doi: 10.1182/blood.v97.7.1990. [DOI] [PubMed] [Google Scholar]
- Kozaki K, Kaminski WE, Tang J, Hollenbach S, Lindahl P, Sullivan C, Yu JC, Abe K, Martin PJ, Ross R, Betsholtz C, Giese NA, Raines EW. Blockade of platelet-derived growth factor or its receptors transiently delays but does not prevent fibrous cap formation in ApoE null mice. Am J Pathol. 2002;161:1395–1407. doi: 10.1016/S0002-9440(10)64415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Masuda J, Raines EW, Gown AM, Katsuda S, Sasahara M, Malden LT, Masuko H, Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990;248:1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Raines EW, Ross R. Platelet-derived growth factor in vivo. Sorg C, editor. Basel: Karger,; Biology of Platelet-Derived Growth Factor. 1993:pp 74–114. [Google Scholar]
- Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Gersuk GM, Westermark B, Mohabeer AJ, Challita PM, Pattamakom S, Pattengale PK. Inhibition of human natural killer cell activity by platelet-derived growth factor (PDGF). III. Membrane binding studies and differential biological effect of recombinant PDGF isoforms. Scand J Immunol. 1991;33:521–532. doi: 10.1111/j.1365-3083.1991.tb02522.x. [DOI] [PubMed] [Google Scholar]
- Daynes RA, Dowell T, Araneo BA. Platelet-derived growth factor is a potent biologic response modifier of T cells. J Exp Med. 1991;174:1323–1333. doi: 10.1084/jem.174.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T, Shimano H, Gotoda T, Harada K, Shimada M, Ohsuga J, Watanabe Y, Kawamura M, Yazaki Y, Yamada N. Expression of platelet-derived growth factor beta receptor on human monocyte-derived macrophages and effects of platelet-derived growth factor BB dimer on the cellular function. J Biol Chem. 1993;268:24353–24360. [PubMed] [Google Scholar]
- Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- Zhou X, Hansson GK. Immunomodulation and vaccination for atherosclerosis. Expert Opin Biol Ther. 2004;4:599–612. doi: 10.1517/14712598.4.4.599. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- Boring LGJ, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest. 1998;101:1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AK, Zhou X, Strandvik B, Hansson GK. Severe hypercholesterolaemia leads to strong Th2 responses to an exogenous antigen. Scand J Immunol. 2004;59:285–293. doi: 10.1111/j.0300-9475.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti A, Kaveri S, Caligiuri G, Bariety J, Hansson GK. Immunoglobulin treatment reduces atherosclerosis in apo E knockout mice. J Clin Invest. 1998;102:910–918. doi: 10.1172/JCI119892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MK, Springer TA. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982;128:1221–1228. [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Wille PT, Philalay J, Blobel CP, Dempsey PJ, Raines EW. Stimulated shedding of VCAM-1 is mediated by TACE (ADAM17). J Biol Chem. 2003;278:37459–37464. doi: 10.1074/jbc.M305877200. [DOI] [PubMed] [Google Scholar]
- Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, Curtiss LK, Witztum JL. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10:325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Raines EW, Ross R, Gold LI, Wight TN. Proteoglycan distribution in lesions of atherosclerosis depends on lesion severity, structural characteristics, and the proximity of platelet-derived growth factor and transforming growth factor-beta. Am J Pathol. 1998;152:533–546. [PMC free article] [PubMed] [Google Scholar]
- Holm T, Nielsen J, Claesson M. CD4+CD25+ regulatory T cells: I. Phenotype and physiology. APMIS. 2004;112:629–641. doi: 10.1111/j.1600-0463.2004.apm1121001.x. [DOI] [PubMed] [Google Scholar]
- Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- Houtkamp MA, van Der Wal AC, de Boer OJ, van Der Loos CM, de Boer PA, Moorman AF, Becker AE. Interleukin-15 expression in atherosclerotic plaques: an alternative pathway for T-cell activation in atherosclerosis? Arterioscler Thromb Vasc Biol. 2001;21:1208–1213. doi: 10.1161/hq0701.092162. [DOI] [PubMed] [Google Scholar]
- Wuttge DM, Eriksson P, Sirsjo A, Hansson GK, Stemme S. Expression of interleukin-15 in mouse and human atherosclerotic lesions. Am J Pathol. 2001;159:417–423. doi: 10.1016/S0002-9440(10)61712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacalano NA, Sanden D, Johnston JA. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat Cell Biol. 2001;3:460–465. doi: 10.1038/35074525. [DOI] [PubMed] [Google Scholar]
- Daynes RA, Araneo BA. Programming of lymphocyte responses to activation: extrinsic factors, provided microenvironmentally, confer flexibility and compartmentalization to T-cell function. Chem Immunol. 1992;54:1–20. [PubMed] [Google Scholar]
- Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H, Lutz M, Rollinghoff M, Bogdan C. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J Immunol. 2001;166:3075–3082. doi: 10.4049/jimmunol.166.5.3075. [DOI] [PubMed] [Google Scholar]
- Zang YC, Samanta AK, Halder JB, Hong J, Tejada-Simon MV, Rivera VM, Zhang JZ. Aberrant T cell migration toward RANTES and MIP-1 alpha in patients with multiple sclerosis. Overexpression of chemokine receptor CCR5. Brain. 2000;123:1874–1882. doi: 10.1093/brain/123.9.1874. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res. 2001;89:E41–E45. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- Egan PJ, Lawlor KE, Alexander WS, Wicks IP. Suppressor of cytokine signaling-1 regulates acute inflammatory arthritis and T cell activation. J Clin Invest. 2003;111:915–924. doi: 10.1172/JCI16156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XQ, Brady G, Iscove NN. Platelet-derived growth factor (PDGF) activates primitive hematopoietic precursors (pre-CFCmulti) by up-regulating IL-1 in PDGF receptor-expressing macrophages. J Immunol. 1993;150:2440–2448. [PubMed] [Google Scholar]
- Dainiak N, Davies G, Kalmanti M, Lawler J, Kulkarni V. Platelet-derived growth factor promotes proliferation of erythropoietic progenitor cells in vitro. J Clin Invest. 1983;71:1206–1214. doi: 10.1172/JCI110869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche F, Raines E, Powell J, Ross R, Adamson J. Platelet-derived growth factor enhances in vitro erythropoiesis via stimulation of mesenchymal cells. J Clin Invest. 1985;76:137–142. doi: 10.1172/JCI111936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daynes RA, Araneo BA. Natural regulators of T-cell lymphokine production in vivo. J Immunother. 1992;12:174–179. doi: 10.1097/00002371-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Wiedmeier SE, Mu HH, Araneo BA, Daynes RA. Age- and microenvironment-associated influences by platelet-derived growth factor on T cell function. J Immunol. 1994;152:3417–3426. [PubMed] [Google Scholar]
- Shure D, Senior RM, Griffin GL, Deuel TF. PDGF AA homodimers are potent chemoattractants for fibroblasts and neutrophils, and for monocytes activated by lymphocytes or cytokines. Biochem Biophys Res Commun. 1992;186:1510–1514. doi: 10.1016/s0006-291x(05)81577-3. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, French WJ, Soriano P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003;1:288–299. doi: 10.1371/journal.pbio.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]