Figure 8-6913.
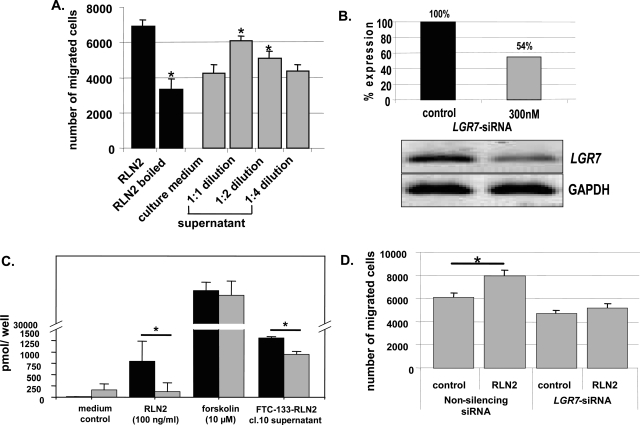
The specificity of the RLN2-LGR7 signaling in mediating the described tumor-promoting activity of RLN2 was shown by reducing the amount of bioactive RLN2 ligands (A) and by decreasing the number of LGR7 transcripts using a specific siRNA-LGR7 construct (B–D). A: Heat-inactivation of 500 ng/ml of RLN2 significantly decreased cellular motility (black columns). Dilution of culture supernatants from FTC-133-RLN2 transfectants also revealed a clear concentration-dependent decrease in motility of FTC-133 (gray columns). FTC-133 exposed to normal culture medium served as negative control. B: After 3 days of incubation with a specific siRNA-LGR7 at 300 nmol/L, FTC-133 displayed an ∼50% reduction in LGR7 transcripts. C: These siRNA-LGR7-treated FTC-133 cells (gray columns) displayed a significant reduction in cAMP response on stimulation with either recombinant human RLN2 or supernatants derived from FTC-133-RLN2 (clone 10) as compared to FTC-133 transfected with the nonsilencing siRNA construct (black columns). D: Nonsilencing siRNA also failed to inhibit the motility-promoting effect of recombinant RLN2 when compared to control cells not exposed to recombinant RLN2. FTC-133 and FTC-238 (not shown) treated with the siRNA-LGR7 did not elicit increased motility in the presence of exogenous RLN2. Thus, the cAMP response toward RLN2 and the motility-enhancing action of (pro)RLN2 were both mediated by the LGR7 signaling pathway in FTC-133 human thyroid carcinoma cells.