Abstract
The progression of chronic hepatitis B (CHB) is related to fibrosis and to the emergence of intrahepatic anomalous vascular structures. Hepatitis B virus (HBV) X protein transactivator (HBx) may play a significant role in both processes. To analyze how HBV induces vascular growth and remodeling in vivo, we assessed the expression of angiopoietin-2 (Ang2) in liver biopsies from CHB patients by reverse transcriptase-polymerase chain reaction, Western blotting, and immunohistochemistry because of the relevant role of Ang2 in vascular development, remodeling, and tumor promotion. In addition, we analyzed the influence of HBx in the expression of Ang2 in HBx-expressing hepatocyte cell lines and in hepatic stellate cells stimulated with conditional medium from HBx-hepatocytes. Ang2 expression was clearly up-regulated at both mRNA and protein levels in the liver of CHB patients, showing an intense staining of inflammatory infiltrates and vascular structures at inflamed portal areas. HBx-expressing hepatocytes and stimulated stellate cells showed a significant induction of Ang2 expression. PI3K inhibitor and antioxidants repressed the 64-kd Ang2 form but further enhanced the inflammation-related 50-kd molecular species. Therefore, HBx could account for the induction of Ang2 observed in CHB, especially the 50-kd form, contributing to pathological angiogenesis and hepatocellular carcinoma progression.
Chronic hepatitis B virus (HBV) infection is directly related to fibrosis progression and cirrhosis, which could ultimately lead to development of hepatocellular carcinoma (HCC).1,2 HBV X protein (HBx) shows multiple biological effects as a viral transactivator, and it has been implicated in tumor invasion and progression.3,4 HBx dual behavior as transcriptional co-activator in the nucleus and signal transduction modulator in the cytoplasm could explain its pleiotropic effects in gene expression despite being unable to bind double-stranded DNA.5 HBx favors many processes that are involved in inflammatory responses and tumor development such as cellular proliferation, apoptosis, metastasis, and angiogenesis.6
In the context of angiogenesis, it has been demonstrated that HBx is able to induce the expression of inducible nitric-oxide synthase (iNOS)7 and vascular endothelial growth factor (VEGF),8 which are actively involved in angiogenesis.9 On the contrary, no information is available about the influence of HBx on the expression of other key factors involved in the formation and stability of vessels, such as angiopoietins. Among the members of this family, angiopoietin-1 (Ang1) and angiopoietin-2 (Ang2) are the best characterized. Both bind the same tyrosine kinase receptor, Tie-2, but only Ang1 is able to trigger its activation,10 being a decisive step in vasculature structural consolidation.11,12 In contrast to the ubiquitous expression of Ang1, Ang2 is closely related to physiological or pathological processes that require vascular network remodeling.13 Ang2 binding to Tie-2 causes vasculature regression because of the destabilization of the interactions between endothelium and basement tissue; however, such an event exerts a marked proangiogenic effect if VEGF is present in the proximity.14,15 HCC is a hypervascular tumor that needs the activation of proangiogenic mechanisms, of which Ang2 has been demonstrated to play a central role.16–18 Hepatic stellate cells (HSCs) constitute the main hypoxia-sensitive cells in HCC. The activation of HSCs in injured liver is a key step in fibrosis development.19 In addition, activated HSCs are associated with the release of proinflammatory mediators and angiogenic factors, such as VEGF, facilitating the migration and growth of hepatoma cells.20–22 Because of the higher risk of HCC development in chronic HBV infection and the central role of Ang2 in promoting tumor formation and progression, we analyzed the expression of Ang2 in liver tissue from CHB patients and its possible regulation by HBx in different hepatic cell lines.
Materials and Methods
Patients and Controls
The diagnostic criteria for CHB patients included in this study (Table 1) were based on the persistent elevation of serum alanine aminotransferase levels as well as on the presence in serum of both hepatitis B surface antigen (HBsAg) and HBV DNA. In addition, all these patients were negative for antibodies against hepatitis C virus and human immunodeficiency virus.
TABLE 1.
Baseline Characteristics of Controls and CHB Patients
| Controls | CHB | |
|---|---|---|
| Sex (M/F) | 2/2 | 8/7 |
| Age (years) | 47.3 ± 18.3 | 45.9 ± 10.3 |
| AST (IU/L) | 35.3 ± 7.4 | 70.5 ± 19.2 |
| ALT (IU/L) | 33.3 ± 2.1 | 133.4 ± 48.7 |
| AP (IU/L) | 52 ± 1.8 | 169.8 ± 35 |
| GGT (IU/L) | 57.3 ± 12.2 | 64.4 ± 25.2 |
| Bilirubin (mg/dl) | 0.7 ± 0.2 | 1.1 ± 0.4 |
| Histological activity index | ||
| Grade | 0.5 ± 0.8 | 2.1 ± 0.5 |
| Stage | 0.4 ± 0.6 | 2.7 ± 1.2 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; AP, alkaline phosphatase; GGT, gammaglutamil transpeptidase. Data are shown as mean ± SD.
Liver Tissue Samples
Biopsies from 15 CHB patients were obtained before antiviral treatment by percutaneous Menghini needle extraction under ecographic control and were either paraffin-embedded, for routine histological examination, or snap-frozen in liquid nitrogen-cooled isopentane and stored at −80°C, for reverse transcriptase-polymerase chain reaction (RT-PCR), immunoblot, or immunohistochemistry assays. The histological severity of CHB was analyzed using the METAVIR semiquantitative scoring system.23 In addition, liver biopsy samples from four patients (negative for HBsAg, anti-HCV, and anti-HIV antibodies) with histologically normal liver, obtained during abdominal surgery for noncomplicated cholelithiasis, were analyzed and used as controls. The Ang2 staining in two HCC samples was also assessed as positive control of the test. Because of the limited amount of tissue samples, biopsies were analyzed for Ang2 expression either by RT-PCR, Western blot, or immunohistochemistry. The study protocol had been approved by the ethical committee of the institution, and signed informed consent was obtained from each patient.
Cell Lines and Culture Conditions
The HBx-expressing hepatic cell line CMX was generated as described24 by stable transfection of the hepatocyte-derived cell line Chang Liver (CCL13; American Type Culture Collection, Manassas, VA) with a pMMTV-X plasmid that expresses the HBV transactivator HBx in a dexamethasone (DX)-inducible manner. Both lines were grown to 60% confluence in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and 2 mmol/L each of streptomycin, glutamine, and penicillin. At this point, HBx expression was stimulated in CMX for 24 hours by replacing the medium with a similar one without fetal calf serum and containing or not DX (CMXDx and CMX, respectively) to a final concentration of 1 μmol/L. This was also performed with the CCL13 line (CCL13Dx and CCL13).
ChangX-34 cells were kindly provided by Dr. Hyeseon Cho (Department of Biochemistry and Molecular Biology, Chronic Inflammatory Disease Research Center, Ajou University School of Medicine, Suwon, South Korea). This subline was established after stably transfecting the human Chang liver cells (CCL13, originally purchased from American Type Culture Collection) with pTetX and pUHD172-1 plasmids.25 Although this subline was originally designed to express HA-tagged HBx in a doxycycline-inducible manner, the basal expression of HBx was substantially maintained (data not shown). These cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin in a humidified CO2 incubator.
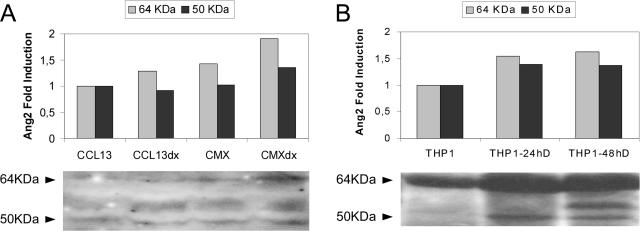
The rat hepatic stellate cells CFSC-2G, kindly provided by Dr. Solis Herruzo (Hospital Doce de Octubre, Madrid, Spain), were maintained with 0% fetal calf serum/Dulbecco’s modified Eagle’s medium until its exposure to the medium from CCL13 (HSC/CCL13) and CMX (HSC/CMX) stimulated or not for 24 hours with DX (conditioned media). The THP-1 promonocyte cell line was used as control because of its described expression of Ang2 isoforms when it is stimulated to differentiation.10 These cells were maintained in RPMI medium supplemented with 10% fetal calf serum and were subjected to 24 or 48 hours of PMA and interleukin (IL)-4 differentiation stimuli.
Inhibition of Intracellular Signaling Pathways
CCL13 and ChangX-34 cultures were pretreated with vehicle (dimethyl sulfoxide), PI3-K signaling inhibitor (LY294002), or MAPK signaling inhibitor (PD98059) or with the antioxidant Ebselen (all purchased from Calbiochem, La Jolla, CA) at 25 μmol/L for 48 hours. Then cells were lysed and extracted for Western blot analysis as described above.
RT-PCR Assays
Total RNA extracts were obtained from cell lines or liver tissues using Ultraspec reactive according to the manufacturer’s protocol (Biotecx Laboratories, Inc., Houston, TX) and were reverse-transcribed (GeneAmp Gold RNA PCR core kit; Applied Biosystems, Foster City, CA) at room temperature for 10 minutes and at 42°C for 60 minutes. The reaction was stopped by heat inactivation, and cDNA products were amplified by PCR with Ang2-specific internal primers Ang2 (sense: 5′-CTGCAAGTGCTGGAGAACATCATGG-3′; anti-sense: 5′-GTGTTCCAAGAGCTGAAGTTC-3′) performing 35 amplification cycles as follows: 30 seconds at 94°C, 30 seconds at 58°C, and 45 seconds at 72°C. The same quantity of cDNA products were amplified by PCR with β-actin-specific primers (sense: 5′-CCCAGAGCAAGAGAGG-3′; anti-sense: 5′-GTCCAGACGCAGGATG-3′) as internal control. The RT-PCR products were separated on agarose gels and visualized by ethidium bromide staining.
Western Blot Analysis
Liver tissue samples and cells were lysed in ice-cold RIPA buffer supplemented with protease inhibitors (Complete; Roche Applied Science, Indianapolis, IN). Whole cell lysates were obtained by subsequent centrifugation at 14,000 rpm for 10 minutes at 4°C. Protein concentrations were determined by the Bradford (Bio-Rad, Hercules, CA) protein assay with bovine serum albumin as standard, and 25 μg of protein extracts were subjected to reducing 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Bio-Rad), which was probed with an Ang2-specific antibody (MAB0983; R&D Systems, Minneapolis, MN) after being blocked with a 7.5% solution of nonfat dry milk dissolved in Tris-HCl-buffered solution (TBS, pH 7.5). Membranes were subsequently TBST (TBS, 0.1% Tween 20) washed, exposed for 45 minutes at room temperature to a secondary goat anti-mouse horseradish peroxidase antibody (Pierce Biotechnology, Rockford, IL), and detected by chemiluminescence (SuperSignal West Pico; Pierce Biotechnology) followed by autoradiography and densitometry analysis.
Immunohistochemical Analysis
Five-μm cryostat liver sections were exposed for 20 minutes to a 1:40 dilution of rabbit serum in TBS to block Fc receptors and incubated or not (negative controls) with an anti-Ang2 antibody (SC-7015; Santa Cruz Biotechnology, Santa Cruz, CA) at a working concentration of 2 μg/ml in a humidified chamber. After three TBS washes, the slides were incubated with a rabbit anti-goat horseradish peroxidase-conjugated antibody (DAKOCytomation, Copenhagen, Denmark) for 45 minutes at room temperature, TBS washed, and exposed to a 3,3′-diaminobenzidine solution (Sigma Fast; Sigma-Aldrich, St. Louis, MO) until color development, counterstained with hematoxylin, and mounted by routine method.
Results
Ang2 Expression Is Enhanced in Liver Samples from CHB Patients
The expression of Ang2 in the liver of CHB patients was clearly induced when compared with control liver tissues.
RT-PCR Studies
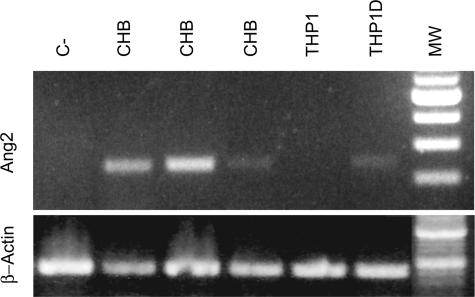
In RT-PCR experiments we could detect the Ang2 mRNA product in the CHB liver tissue samples tested (n = 3), similar to that observed in THP1 cell line stimulated to differentiation. Ang2 mRNA product was clearly absent in controls (Figure 1).
FIGURE 1.
Ang2 mRNA expression is induced in the liver of CHB patients. RT-PCR analysis of Ang2 expression in liver samples from patients and controls shows that a direct relationship exists in HBV-infected individuals. Ang2 is enhanced in 48-hour stimulated THP1 cells (THP1D).
Western Blot Studies
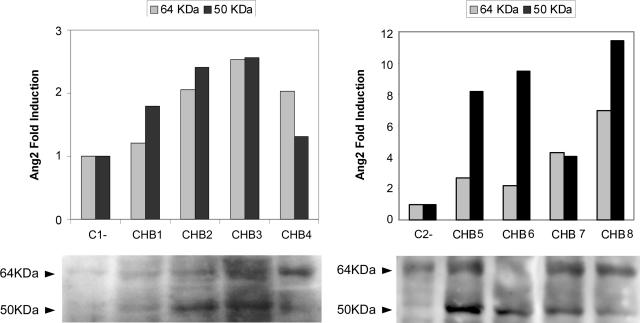
In addition, we observed a significant induction in Ang2 protein expression in the CHB specimens analyzed (n = 8) by Western blot (64 kd), which reached up to sevenfold increase, and a noticeable specific immunoreactivity at 50 kd, which was strongly induced in CHB patients (Figure 2).
FIGURE 2.
The expression of Ang2 protein is enhanced in the liver of CHB patients. Representative Ang2 Western blot of CHB and control (C1 and C2) liver samples, densitometrically analyzed, shows the induction of Ang2 reactivity at 64 and 50 kd in CHB liver protein extracts.
Immunohistochemical Studies
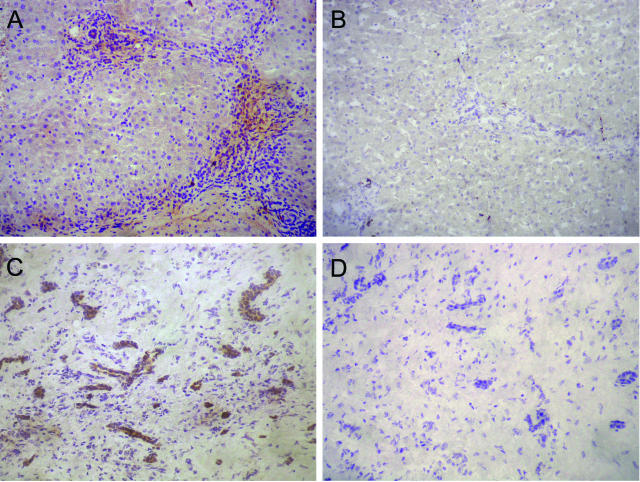
Immunoperoxidase analysis of Ang2 expression in tissue samples of CHB patients (n = 4) showed a clear staining at inflamed portal and periportal areas in the inflammatory infiltrates that extended along the fibrotic septa (Figure 3A). In addition, scattered parenchymal staining was also observed (Figure 3A and data not shown). In control liver tissues the expression of Ang2 was totally absent (Figure 3B). The Ang2 reactivity in HCC samples was mostly associated with vascular structures (Figure 3C), whereas, as expected, no reactivity was detected when anti-Ang2 primary antibody was not included (Figure 3D).
FIGURE 3.
Ang2 expression pattern in liver tissue samples. Immunoperoxidase analysis of Ang2 protein expression in hepatic specimens shows a marked positive staining in the inflamed portal tracts of CHB liver tissue sections (A), mostly related to the mononuclear inflammatory infiltrate. Ang2 expression was negative in control normal liver (B) but clearly positive in vascular structures of HCC samples (C); as expected, an absence of immunoreactivity was observed in the same HCC sample (D) without using the primary anti-Ang2-specific antibody SC-7015. Original magnifications, ×200.
HBx Protein Promotes Ang2 Expression in Hepatic Cell Lines and in Stimulated Stellate Cells
RT-PCR Studies
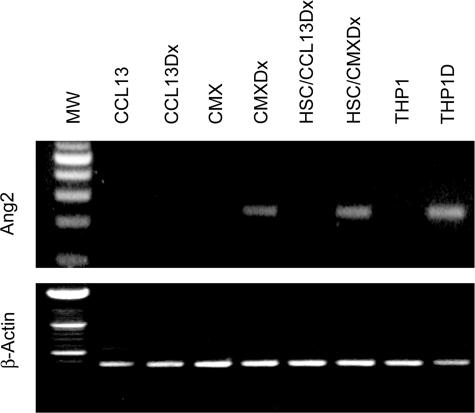
To investigate whether HBx could induce the expression of Ang2 we performed RT-PCR analysis of the Ang2 transcript in the hepatocyte-derived cell line CCL13 and in the HBx-expressing cells CMX, with or without DX. In addition, the induction of Ang2 expression in HSCs was assessed after the exposure of these cells to conditioned medium from CMX or CCL13 cells treated with DX. We observed that HBx protein promoted Ang2 mRNA synthesis in CMX cells and in the HSCs exposed to CMX-conditioned medium (Figure 4, top). Moreover, by 48 hours PMA and IL-4 differentiation stimulus induced the Ang2 mRNA expression in THP1 monocyte precursor cells. Equal loading of total RNA was confirmed by the analysis of β-actin mRNA transcripts (Figure 4, bottom).
FIGURE 4.
Angiopoietin-2 mRNA expression in hepatic cell lines. Ang2 mRNA expression is directly related to enhanced HBx-expressing or HBx-conditioned hepatic lines (CMXDx and HSC/CMXDx, respectively). The promonocyte cell line THP1 expresses Ang2 mRNA after 48 hours of IL-4 and PMA differentiation stimuli (THP1 and THP1D).
Western Blot Studies
The immunoblotting protein analysis revealed higher intensity of Ang2 signal at 64 kd in cells that express HBx (Figure 5A), similar to stimulated THP1 cell line (Figure 5B). Of note, the induction of a 50-kd reactivity observed either in HBx-expressing cells or specifically in stimulated THP1 cells (Figure 5, A and B) resemble that observed exclusively in CHB protein extracts (Figure 2).
FIGURE 5.
Expression of Ang2 protein in hepatic and promonocyte cell lines. The immunoblots demonstrate enhanced Ang2 immunoreactivity in HBx-expressing cells at both 64 and 50 kd (A) as observed in THP1 cells stimulated to differentiation (B). Figure shows representative Western blots of several experiments with the densitometric analysis.
Signal Transduction Pathways Implicated in HBx-Induced Ang2 Expression
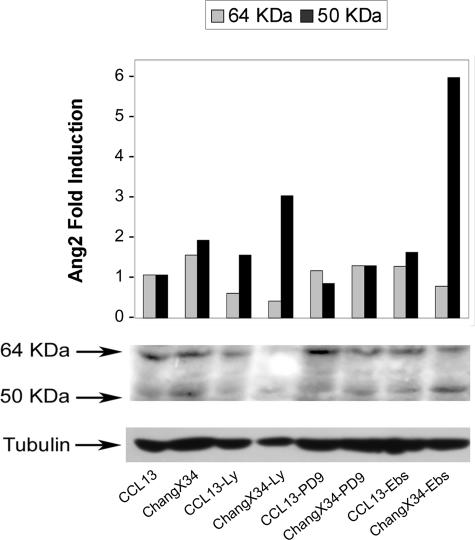
Given that it has been described that HBx regulates several signal transduction pathways we analyzed which of these could be involved in the induction of Ang2 by this viral protein. We tested the effect of the PI3-K and MAPK inhibitors (LY294002 and PD98059, respectively) as well as the antioxidant Ebselen in ChangX-34 cells, which expressed basal levels of HBx protein. As shown for CMX cells in Figure 5, the expression of both Ang2 forms was also increased in ChangX-34 compared with parental CCL13 cells. The inhibition of PI3K or reactive oxygen species production in ChangX-34 cells resulted in a significant decrease of 64-kd form. In contrast, the expression of the 50-kd form, which has been related to inflammation and tumor progression, was further enhanced in the presence of LY294002 and Ebselen in the HBx-expressing cells, but not in parental cells (Figure 6). The MAPK inhibitor PD98059 prevented the HBx-mediated induction of both Ang2 isoforms (Figure 6).
FIGURE 6.
Inhibition of cellular signal transduction pathways. HBx-expressing or not expressing hepatocyte-derived cell lines (CCL13 and ChangX-34, respectively) were treated to block PI3K or MAPK signaling pathways (Ly and PD9, respectively) as well as to inhibit the synthesis of reactive oxygen species with the superoxide dismutase mimetic Ebselen (Ebs). Figure shows the differential regulation of both Ang2 isoforms expression (64 and 50 kd).
Discussion
Vasculature networks represent a structural interface of solutes and cellular interchange between the blood and surrounding tissues in relation to microenvironmental demands such as oxygen, nutrient or immune supply, and removal of waste products. Therefore, the molecular mechanisms controlling vascular growth and integrity must be tightly regulated because their imbalance could be a pathogenic cause of multiple diseases.26–28 Angiopoietins are major mediators of angiogenic control by their interaction with the endothelial tyrosine kinase receptor Tie2. Ang2 impairs Tie2 phosphorylation triggered by constitutive Ang1 interaction, acting as a natural antagonist that results in structural leakage of vasculature. This event is an essential step for vascular dynamic response to environmental stimuli. Thus, beyond the widely described morphogenetic roles of angiopoietins, there is increasing evidence relating angiopoietins with the immune regulation due to their functional implications on vessel integrity and permeability.29 Inflammatory response requires endothelial cell activation that leads leukocyte firm adhesion and extravasation to injured tissue.30 This process has been recently shown to be promoted by Ang2 in experiments using suboptimal doses of tumor necrosis factor-α.31 Moreover, Ang1-Tie2-derived signaling through PI3-K activation promotes endothelial cell survival, resulting in vessel structural stabilization, and blocks NF-κB proinflammatory effects that are reverted by Ang2 antagonist function.
As described, HBV infection results in a chronic inflammatory disease in a substantial percentage of patients, and it is highly related to HCC development. Among HBV proteins, HBx has arisen as the main one responsible of the angiogenic and immune side effects related to CHB disease. Previous studies have demonstrated that HBx protein is able to induce the production of some angiogenesis-related molecules such as NO and VEGF.7–9 However, to our knowledge, there is no evidence of the contribution of HBx in Ang2 expression. Most HBx-mediated proangiogenic effects could be related to its ability to stabilize and facilitate the transactivation function of the hypoxia inducible factor 1α (HIF-1α).32–34 It is known that HIF-1α regulates the expression of multiple genes involved in angiogenesis processes such as VEGF and matrix metalloproteinase 2 (MMP2),35,36 which are also up-regulated by HBx.4,8 COX2 mediates the activation of MMP2 by HBx, and, independently, it has been described that Ang2 also activates MMP2.37 In addition, it has been recently described that the in vitro and in vivo Ang2 mRNA regulation mediated by COX2 in HCC cells could account for anti-tumor properties of COX2 inhibitors.38 There is emerging evidence that several proinflammatory cytokines, such as interferon-γ, IL-1β, and tumor necrosis factor, stabilize HIF-1α, even in the absence of hypoxia,39,40 and the same cytokines are able to induce HBx expression. Herein we demonstrate for the first time that HBx stimulates Ang2 mRNA synthesis in hepatocyte cell lines under normoxic conditions. In addition, we show the influence of mediators generated by HBx-expressing cells in the induction of Ang2 mRNA expression by HSCs that was also observed in the analyzed CHB liver samples. There was also a remarkable rise in Ang2 protein level expression related to HBx-expressing cells, stimulated THP1 cells, or CHB liver tissue in which we could also describe a noticeable induction of an additional Ang2 form of 50 kd. This finding is clearly in agreement with the induction of Ang2-443 isoform observed in several inflammatory and tumor diseases,10 and emphasizes the requirement of in depth study of the functional relevance of Ang2 isoforms in the pathophysiology and progression of CHB and other angiogenic related diseases as have been described for VEGF isoforms.41 In this regard, the blockage of PI3-K or reactive oxygen species synthesis pathways produced an intense induction of inflammatory-related Ang2 protein expression by ChangX-34 HBx-expressing cells opposite to the angiogenic 64-kd form. Therefore, such differential regulation of Ang2 isoforms could involve relevant pathophysiological implications and could directly explain the proinflammatory and proangiogenic imbalance of HBV-related chronic liver disease. In addition, evidence exists of the decisive role of different signal transduction pathways in the promotion and evolution of diverse neoplasms.42–44
Regarding the tissue distribution of Ang2 it should be noted that in CHB liver tissue the main Ang2 staining is related to the inflammatory infiltrates at inflamed periportal spaces and fibrosis septa edges. HSCs play a central role in fibrogenesis and tumor-related angiogenesis because they are the main source of cytokines and inflammatory mediators that regulate the hepatic intercellular crosstalk in both autocrine and paracrine manners. Thus, progressive chronic HBV disease could favor the maintenance of multiple pathological pathways such as inflammation and carcinogenesis by contributing to the angiogenic process through the induction and regulation of Ang2 isoform expression by HBx. HBV infection often results in a chronic liver injury because of ineffective immune response and it is directly related to fibrosis progression. In this regard, we have clearly observed the activation of HSCs by HBx by analyzing the expression of smooth muscle actin (α-SMA) and collagen type 1-α in HSCs after their exposure to CMX-conditioned media (our unpublished data). HSCs are special liver pericytes, cells that are major endothelial cell regulators by the release, among others, of Ang1.
In the present study we have appreciated the Ang2 expression by HSCs in response to mediators generated by HBx-expressing hepatic cells priming inflammation. Therefore, Ang2 could be the linking factor among the inflammatory, angiogenic, and fibrogenic phenomena associated to this and other chronic inflammatory diseases and could explain why many anti-angiogenic agents are both anti-inflammatory and anti-fibrogenic.45 Moreover, such a proangiogenic background and vascular exchange permissiveness could clearly facilitate HCC development and the spreading of tumor cells. Therefore, the direct anti-angiogenic effect resulting from a specific inhibitor of Ang2 may indicate its possible usefulness in future therapeutic designs.46
In conclusion, the present study clearly indicates that the angiogenic activation process is an important mechanistic step in the pathology of chronic inflammatory diseases such as CHB. The analysis of Ang2 expression, and in particular of its different isoforms, may help to understand the pathophysiological pathways that lead the progression of chronic liver injury to end-stage cirrhosis and HCC development. We describe for the first time the ability of HBV, through HBx, to promote Ang2 expression in liver tissue of chronically infected patients. More meaningful investigation will require study of primary cells and higher number of hepatic samples to reinforce this novel finding and to define its prognostic implications in CHB patients.
Footnotes
Address reprint requests to Ricardo Moreno-Otero, Unidad de Hepatología (Planta 3), Hospital Universitario de la Princesa, Diego de León 62, E-28006, Madrid, Spain. E-mail: rmoreno.hlpr@salud.madrid.org.
Supported by SAF (2004/07885 to R.M.-O. and 2004/07855 to M.L.-C.) and Instituto de Salud Carlos III (C03/02 to R.M.-O. and M.L.-C.).
References
- Beasley RP, Lin CC, Hwang LY, Chien CS. Hepatocellular carcinoma and hepatitis B virus. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- Kim CM, Koike K, Saito I, Miyamura T, Jay G. Hbx gene of hepatitis-B virus induces liver-cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- Lara-Pezzi E, Gomez-Gaviro MV, Galvez BG, Mira E, Iniguez MA, Fresno M, Martinez-A C, Arroyo AG, Lopez-Cabrera M. The hepatitis B virus X protein promotes tumor cell invasion by inducing membrane-type matrix metalloproteinase-1 and cyclooxygenase-2 expression. J Clin Invest. 2002;110:1831–1838. doi: 10.1172/JCI200215887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris G, Siddiqui A. Regulatory mechanisms of viral hepatitis B and C. J Biosci. 2003;28:311–321. doi: 10.1007/BF02970150. [DOI] [PubMed] [Google Scholar]
- Maguire HF, Hoeffler JP, Siddiqui A. HBV X protein alters the DNA binding specificity of CREB and ATF-2 by protein-protein interactions. Science. 1991;252:842–844. doi: 10.1126/science.1827531. [DOI] [PubMed] [Google Scholar]
- Majano PL, Garcia-Monzon C, Lopez-Cabrera M, Lara-Pezzi E, Fernandez-Ruiz E, Garcia-Iglesias C, Borque MJ, Moreno-Otero R. Inducible nitric oxide synthase expression in chronic viral hepatitis. Evidence for a virus-induced gene upregulation. J Clin Invest. 1998;101:1343–1352. doi: 10.1172/JCI774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Lee YM, Bae SK, Murakami S, Yun Y, Kim KW. Human hepatitis B virus X protein is a possible mediator of hypoxia-induced angiogenesis in hepatocarcinogenesis. Biochem Biophys Res Commun. 2000;268:456–461. doi: 10.1006/bbrc.2000.2093. [DOI] [PubMed] [Google Scholar]
- Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185–1195. doi: 10.1002/hep.20193. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim JH, Ryu YS, Jung SH, Nah JJ, Koh GY. Characterization and expression of a novel alternatively spliced human angiopoietin-2. J Biol Chem. 2000;275:18550–18556. doi: 10.1074/jbc.M910084199. [DOI] [PubMed] [Google Scholar]
- Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol. 2001;2:257–267. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Mori M, Sakamoto Y, Makuuchi M, Sugimachi K, Wands JR. Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J Clin Invest. 1999;103:341–345. doi: 10.1172/JCI4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi N, Shimizu H, Ohtsuka M, Wakabayashi Y, Ito H, Kimura F, Yoshidome H, Kato A, Nukui Y, Miyazaki M. Angiopoietins and Tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology. 2003;37:1105–1113. doi: 10.1053/jhep.2003.50204. [DOI] [PubMed] [Google Scholar]
- Torimura T, Ueno T, Kin M, Harada R, Taniguchi E, Nakamura T, Sakata R, Hashimoto O, Sakamoto M, Kumashiro R, Sata M, Nakashima O, Yano H, Kojiro M. Overexpression of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. J Hepatol. 2004;40:799–807. doi: 10.1016/j.jhep.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology. 2000;31:141–148. doi: 10.1002/hep.510310122. [DOI] [PubMed] [Google Scholar]
- Theret N, Musso O, Turlin B, Lotrian D, Bioulac-Sage P, Campion JP, Boudjema K, Clement B. Increased extracellular matrix remodeling is associated with tumor progression in human hepatocellular carcinomas. Hepatology. 2001;34:82–88. doi: 10.1053/jhep.2001.25758. [DOI] [PubMed] [Google Scholar]
- Olaso E, Salado C, Egilegor E, Gutierrez V, Santisteban A, Sancho-Bru P, Friedman SL, Vidal-Vanaclocha F. Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology. 2003;37:674–685. doi: 10.1053/jhep.2003.50068. [DOI] [PubMed] [Google Scholar]
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C: the METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- Chirillo P, Pagano S, Natoli G, Puri PL, Burgio VL, Balsano C, Levrero M. The hepatitis B virus X gene induces p53-mediated programmed cell death. Proc Natl Acad Sci USA. 1997;94:8162–8167. doi: 10.1073/pnas.94.15.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C, Cho H, Kim SJ, Lee JH, Park SY, Chan GK, Cho H. Mitotic aberration coupled with centrosome amplification is induced by hepatitis B virus X oncoprotein via the Ras-mitogen-activated protein/extracellular signal-regulated kinase-mitogen-activated protein pathway. Mol Cancer Res. 2004;2:159–169. [PubMed] [Google Scholar]
- Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar VV, Stalmans I, Mattot V, Perriard JC, Dewerchin M, Flameng W, Nagy A, Lupu F, Moons L, Collen D, D’Amore PA, Shima DT. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Sanz-Cameno P, Garcia-Buey L, Martin-Vilchez S, Lopez-Cabrera M, Moreno-Otero R. Evidence of angiogenesis in primary biliary cirrhosis: an immunohistochemical descriptive study. J Hepatol. 2005;42:124–131. doi: 10.1016/j.jhep.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Imhof BA, Aurrand-Lions M. Angiogenesis and inflammation face off. Nat Med. 2006;12:171–172. doi: 10.1038/nm0206-171. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- Moon EJ, Jeong CH, Jeong JW, Kim KR, Yu DY, Murakami S, Kim CW, Kim KW. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 2004;18:382–384. doi: 10.1096/fj.03-0153fje. [DOI] [PubMed] [Google Scholar]
- Yoo YG, Oh SH, Park ES, Cho H, Lee N, Park H, Kim DK, Yu DY, Seong JK, Lee MO. Hepatitis B virus X protein enhances transcriptional activity of hypoxia-inducible factor-1alpha through activation of mitogen-activated protein kinase pathway. J Biol Chem. 2003;278:39076–39084. doi: 10.1074/jbc.M305101200. [DOI] [PubMed] [Google Scholar]
- Pichiule P, Chavez JC, LaManna JC. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J Biol Chem. 2004;279:12171–12180. doi: 10.1074/jbc.M305146200. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. J Clin Invest. 2001;108:39–40. doi: 10.1172/JCI13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolmede K, Durand de Saint Front V, Galitzky J, Lafontan M, Bouloumie A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3–F442A adipocytes. Int J Obes Relat Metab Disord. 2003;27:1187–1195. doi: 10.1038/sj.ijo.0802407. [DOI] [PubMed] [Google Scholar]
- Hu B, Guo P, Fang Q, Tao HQ, Wang D, Nagane M, Huang HJ, Gunji Y, Nishikawa R, Alitalo K, Cavenee WK, Cheng SY. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci USA. 2003;100:8904–8909. doi: 10.1073/pnas.1533394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Wands JR, Arii S. Induction of angiopoietin-2 gene expression by COX-2: a novel role for COX-2 inhibitors during hepatocarcinogenesis. J Hepatol. 2006;44:233–235. doi: 10.1016/j.jhep.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Scharte M, Han X, Bertges DJ, Fink MP, Delude RL. Cytokines induce HIF-1 DNA binding and the expression of HIF-1-dependent genes in cultured rat enterocytes. Am J Physiol. 2003;284:G373–G384. doi: 10.1152/ajpgi.00076.2002. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem. 1997;272:7151–7158. doi: 10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- Cerimele F, Battle T, Lynch R, Frank DA, Murad E, Cohen C, Macaron N, Sixbey J, Smith K, Watnick RS, Eliopoulos A, Shehata B, Arbiser JL. Reactive oxygen signaling and MAPK activation distinguish Epstein-Barr virus (EBV)-positive versus EBV-negative Burkitt’s lymphoma. Proc Natl Acad Sci USA. 2005;102:175–179. doi: 10.1073/pnas.0408381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- Arbiser JL. Molecular regulation of angiogenesis and tumorigenesis by signal transduction pathways: evidence of predictable and reproducible patterns of synergy in diverse neoplasms. Semin Cancer Biol. 2004;14:81–91. doi: 10.1016/j.semcancer.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52:237–268. [PubMed] [Google Scholar]
- White RR, Shan S, Rusconi CP, Shetty G, Dewhirst MW, Kontos CD, Sullenger BA. Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for angiopoietin-2. Proc Natl Acad Sci USA. 2003;100:5028–5033. doi: 10.1073/pnas.0831159100. [DOI] [PMC free article] [PubMed] [Google Scholar]