Figure 3.
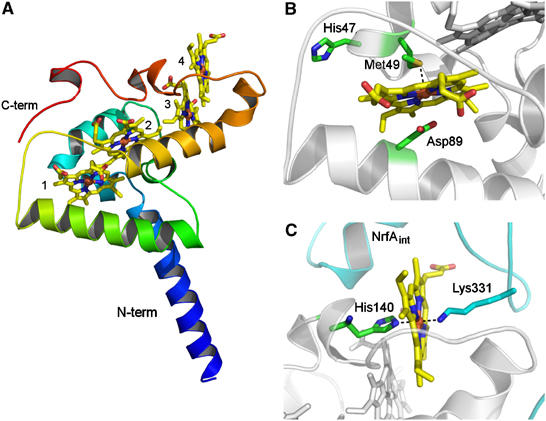
(A) Overall fold of NrfH. The polypeptide chain is ramp-coloured from blue (N-terminal) to red (C-terminal). The four c-type haems are shown in stick rendering and coloured by atom type (iron—brown, oxygen—red, nitrogen—blue, carbon—yellow). The two longer α-helices displayed in blue and green are inserted in the membrane. The haems, numbered according to their attachment to the protein, are arranged in a di-haem parallel motif, where the two pairs of haems are perpendicularly packed. (B) Zoomed view of haem 1 showing the proximal ligand, Met49 (∼2.8 Å distance from Fe), Asp89 (∼3 Å distance from Fe) and His47 from the CXXCHXM motif. These residues show C atoms in green, N in blue, O in red and S in gold. (C) Zoomed view of haem 4 showing its coordination (proximal ligand is His140 and distal one is Lys331 from an internal NrfA subunit, whose carbon atoms are coloured in cyan).
