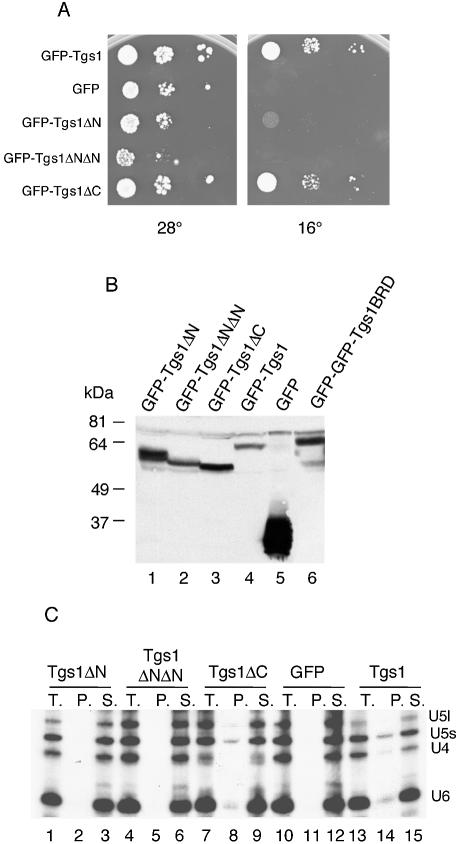
Figure 5.
Functional properties of Tgs1 deletion mutants. (A) Growth assay. Exponentially growing cultures of yeast cells carrying the indicated constructs were serially diluted, spotted on plates and incubated at 16 and 28°C. In contrast to the wild-type and the Tgs1ΔC fusion proteins, the other constructs were unable to restore growth of the tgs1::KAN deletion strain. (B) Western analysis of GFP–Tgs1 mutant fusion proteins. Equivalent amounts of cell extracts prepared from wild-type strains carrying the indicated GFP–Tgs1 alleles were fractionated by SDS–PAGE and immunoblotted with anti-GFP antibodies. Control extract was made from a wild-type strain carrying the GFP vector alone. The Tgs1BRD allele was fused to a GFP–GFP dimer reporter protein which explains the lower mobility of the fusion protein. The other low mobility bands correspond to anti-GFP antibodies cross-reacting polypeptides. (C) Immunoprecipitation experiments. Whole-cell extracts were prepared from tgs1::KAN cells expressing the indicated GFP fusion proteins grown at 28°C and the snRNPs were immunoprecipitated with anti-m3G antibodies. RNA was extracted from the supernatants (S.), from the pellets (P.) and from equivalent aliquots of the total lysates (T.), separated on denaturing polyacrylamide gels, and subjected to northern analysis. Hybridization was performed with probes specific for the yeast U4, U5 and U6 snRNAs.