Figure 2.
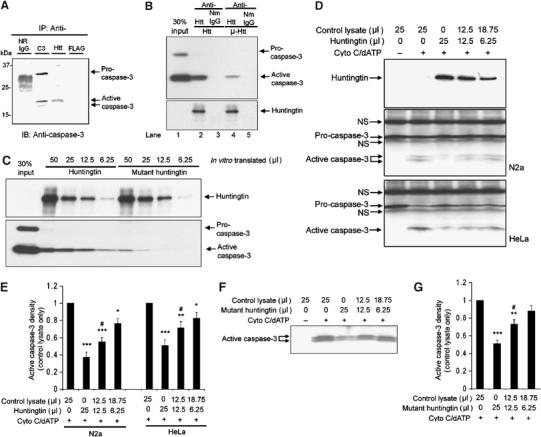
(A) Wild-type huntingtin interact with activated caspase-3. Immunoprecipitation was performed in lysates from N2a cells as described in the Materials and methods section. Caspase-3 antibody (Cell Signal 9662) binds both pro- and active caspase-3. Normal rabbit IgG (N.R.IgG) and mouse anti-FLAG (mouse IgG1) were used as controls. Huntingtin antibody (MAB2166) co-immunoprecipitates active caspase-3. (B) Immunoprecipitation of wild-type huntingtin and mutant huntingtin with active recombinant caspase-3. The full-length huntingtin and mutant huntingtin were first purified from in vitro-translated lysates (50 μl) using conjugated antibodies: anti-huntingtin antibody (MAB 2166) or normal mouse IgG (NmIgG). The purified proteins were then incubated with purified recombinant caspase-3 (2 μg). The bound caspase-3 was detected by Western blot. Blot of anti-huntingtin showed in vitro-translated huntingtin. (C) Western blot of caspase-3 demonstrates different binding affinities of wild-type huntingtin (in an in vitro-translated volume of 50, 25, 12.5, and 6.25 μl) and mutant huntingtin (in an in vitro-translated volume of 50, 25, 12.5, and 6.25 μl) with caspase-3. (D) Western blot for huntingtin and caspase-3 in N2a and HeLa cytosolic extracts following treatment for 1 h with 10 μM cytochrome c and 1 mM dATP at 37°C, with or without in vitro-translated full-length huntingtin. The position of procaspase-3 and active caspase-3 are identified by arrows. Procaspase-3 was mixed with nonspecific bands from in vitro translation reagents (indicated by arrows with ‘NS'. NS=nonspecific band). One representative result of six individual experiments is shown. (E) Densitometric analysis of active caspase-3 signal in Western blot of N2a and HeLa cytosolic extracts with or without in vitro-translated full-length huntingtin as shown in (D). Data are mean±s.e.m. Error bars indicate s.e.m. n=6. ***P<0.001, **P<0.01, *P<0.05 versus control without adding huntingtin. #P<0.05 versus group adding 25 μl of huntingtin. (F) Western blot for caspase-3 in N2a cytosolic extracts following treatment for 1 h with 10 μM cytochrome c and 1 mM dATP at 37°C, with or without in vitro-translated mutant full-length huntingtin. One representative result of six individual experiments is shown. (G) Densitometric analysis of active caspase-3 signal in Western blots as shown in (F). Data are mean±s.e.m. Error bars indicate s.e.m. n=6. ***P<0.001, **P<0.01 versus control without adding mutant huntingtin. #P<0.05 versus group adding 25 μl of mutant huntingtin.
