Figure 6.
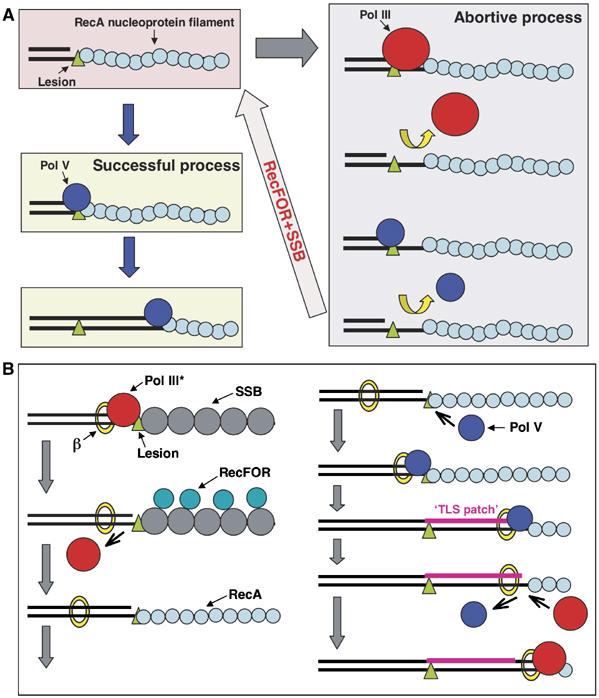
Model for TLS. (A) Interference between Pol III and Pol V. The results presented in this paper suggest that when a RecA/ATP filament is formed along the single-stranded DNA located downstream of a replication blocking lesion, the 3′-OH end of the primer can bind either Pol V or Pol III. When Pol V binds, TLS proceeds successfully as described previously (Fujii and Fuchs, 2004; Fujii et al, 2004). In contrast, binding of Pol III is not only an abortive event for the TLS reaction per se, but it also appears to locally perturb the structure of the 3′-tip of the RecA filament, thereby preventing successful binding of Pol V. Interestingly, our results show that both RecFOR and SSB proteins are able to rapidly ‘reconstruct' the RecA filament for efficient Pol V-mediated lesion bypass, thus counteracting the adverse effect of Pol III on the bypass reaction. (B) Integrated TLS model: this scheme brings together the current data for the bypass of lesion by Pol V in E. coli. Upon reaching a replication block, Pol III dissociates leaving the beta-clamp on the template, while the replication fork helicase keeps moving, thus creating a region of single-stranded DNA downstream of the lesion (Higuchi et al, 2003; Pages and Fuchs, 2003; McInerney and O'Donnell, 2004). Owing to the higher affinity for ssDNA of SSB compared with RecA, SSB will bind first, thus preventing RecA filament formation. The RecFOR mediator proteins are essential for the formation of a RecA filament. Upon binding to the 3′-OH of the primer, Pol V will form a relatively stable initiation complex via a dual interaction with the beta-clamp and the tip of the RecA filament. Synthesis of a ‘TLS patch' in excess of five nucleotides will allow Pol III to resume synthesis. We envision the process of TLS as a gap-filling reaction that occurs ‘behind' the replication fork possibly involving free Pol III core rather than the Pol III holoenzyme (Lehmann and Fuchs, 2006).
