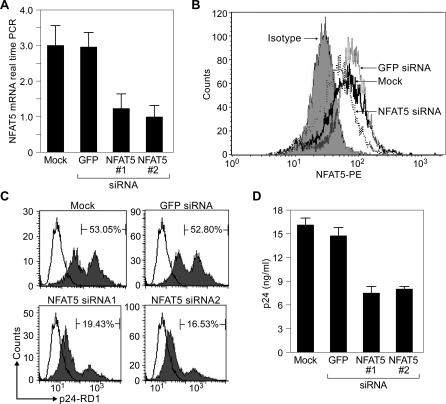
Figure 5. NFAT5 Plays an Important Functional Role in HIV-1 Replication in HeLa-CD4 Cells.
(A) Real-time PCR showing that HeLa-CD4 cells constitutively express NFAT5 mRNA. Using siRNA-targeting NFAT5, we were able to reduce NFAT5 mRNA levels significantly (p < 0.01) compared with that of GFP siRNA or mock-transfected cells. To confirm that NFAT5 knockdown was specific, we used two NFAT5 siRNAs (NFAT5#1 and NFAT#2) targeting two different exons of the NFAT5 gene. We chose the sequence siRNA to GFP because this sequence has been validated and shown to have no nonspecific effects upon HIV replication [46,58]. Results show the average of four independent experiments.
(B) Flow cytomety analysis of intracellular NFAT5 levels in HeLa-CD4 cells. A representative experiment of a flow cytometry analysis using a monoclonal antibody to NFAT5 demonstrated that intracellular NFAT5 protein levels were lower in the cells transfected with NFAT5 siRNA than those that were transfected with GFP siRNA or mock transfected. The experiment is representative of four independent experiments.
(C) Flow cytometry analysis of intracellular p24 levels in HIV-1LAI infected HeLa-CD4 cells. Intracellular HIV-1 levels were determined using a p24-RD1 conjugate in a representative experiment. Three days following virus infection, intracellular p24 was lower in cells transfected with NFAT5 siRNA targeting two different exons of NFAT5 (NFAT5#1 and NFAT5#2; 33.3% and 36.27% inhibition, respectively) than in cells transfected with a GFP control siRNA or mock transfected. This experiment is representative of four independent experiments.
(D) Cell-free p24 production in supernatants of HIV-1LAI infected HeLa-CD4 cells. HIV-1 replication as assessed by p24 ELISA is significantly (p < 0.04) lower in cellular cultures transfected with NFAT5 siRNA targeting two different exons of NFAT5 (NFAT5#1 and NFAT5#2) than in cells mock transfected or transfected with the GFP control siRNA.