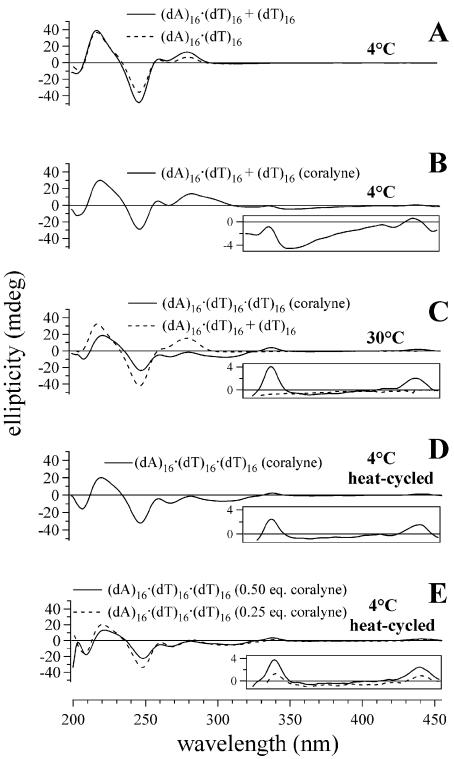
Figure 1.
CD spectra of triplex and duplex 16mer DNA samples demonstrating that complete triplex (dA)16·(dT)16·(dT)16 formation requires heating of the DNA sample in the presence of coralyne. (A) Spectra of duplex (dA)16·(dT)16 and (dA)16·(dT)16 + (dT)16 (unstable triplex) samples at 4°C. (B) Spectrum of (dA)16·(dT)16 + (dT)16 in the presence of coralyne at 4°C. (C) Spectrum of (dA)16·(dT)16 + (dT)16 at 30°C without coralyne and triplex (dA)16·(dT)16·(dT)16 spectrum with coralyne at 30°C. (D) CD spectrum acquired at 4°C of (dA)16·(dT)16·(dT)16 after being heated to 75°C with coralyne. (E) Spectra of triplex (dA)16·(dT)16·(dT)16 at 4°C after heat cycling with 0.25 and 0.5 molar equivalents of coralyne, respectively. DNA concentrations were 55 µM base pair or base triplet, respectively. Coralyne concentration, in samples containing coralyne, was 0.25 molar equivalents per base pair or base triplet, respectively, except as stated in (E).