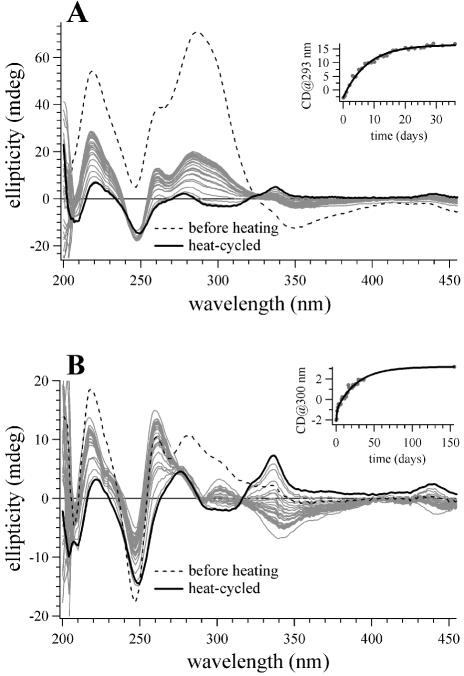
Figure 6.
CD spectra of (dA)32·(dT)32 and poly(dA)·poly(dT) samples with coralyne that illustrate the approach of these samples to equilibrium at 4°C after being heat cycled into the disproportioned state. (A) Spectra of the (dA)32·(dT)32 sample with coralyne at 4°C. The dashed line is the spectrum acquired immediately after the addition of coralyne at 4°C, before sample heat cycling. The solid black line is the spectrum acquired immediately after the sample was heated to 75°C and cooled back to 4°C. Spectra drawn in gray were acquired at selected time intervals after the sample was heat cycled. After heat cycling, the sample was constantly maintained at 4°C. (Inset) A plot of the best fit exponential function of the CD signal at 293 nm for the (dA)32·(dT)32 sample with coralyne at 4°C as a function of time after sample heat cycling. (B) Spectra of the poly(dA)·poly(dT) sample with coralyne at 4°C. The dashed line is the spectrum acquired immediately after the addition of coralyne at 4°C, before sample heat cycling. The solid black line is the spectrum acquired immediately after the sample was heated to 95°C and cooled back to 4°C. Spectra drawn in gray were acquired at selected time intervals after the sample was heat cycled. After heat cycling, the sample was constantly maintained at 4°C. (Inset) A plot of the best fit double exponential function of the CD signal at 300 for the poly(dA)· poly(dT) sample with coralyne at 4°C nm as a function of time after heat cycling. DNA concentration was 55 µM base pair. Coralyne concentration was 0.25 molar equivalents per base pair for both samples.