Abstract
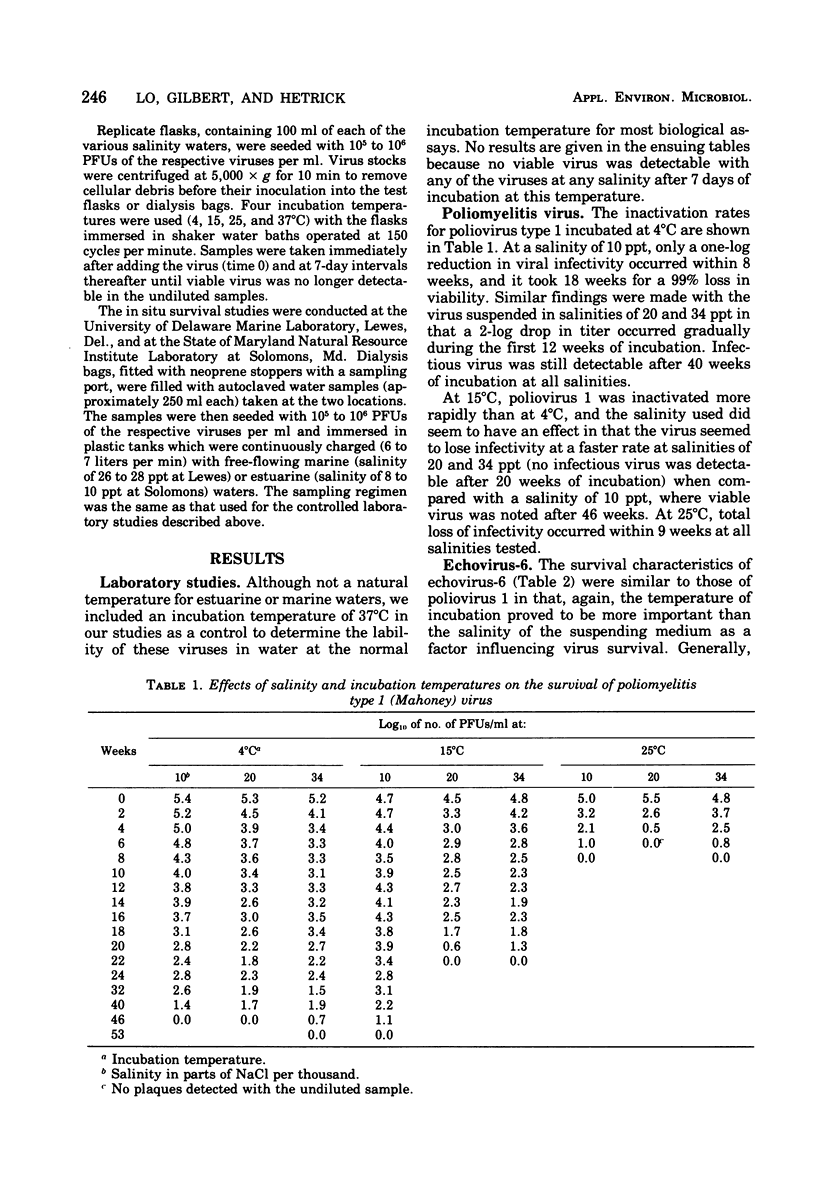
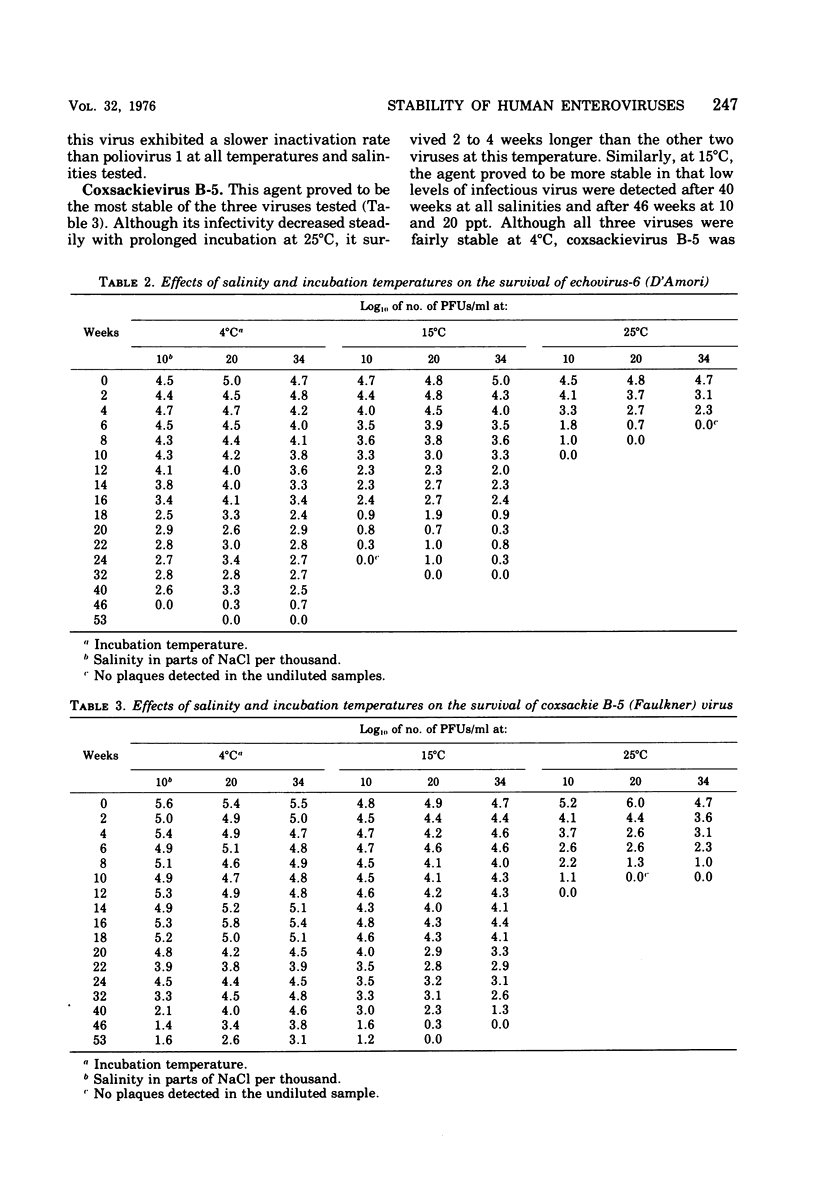
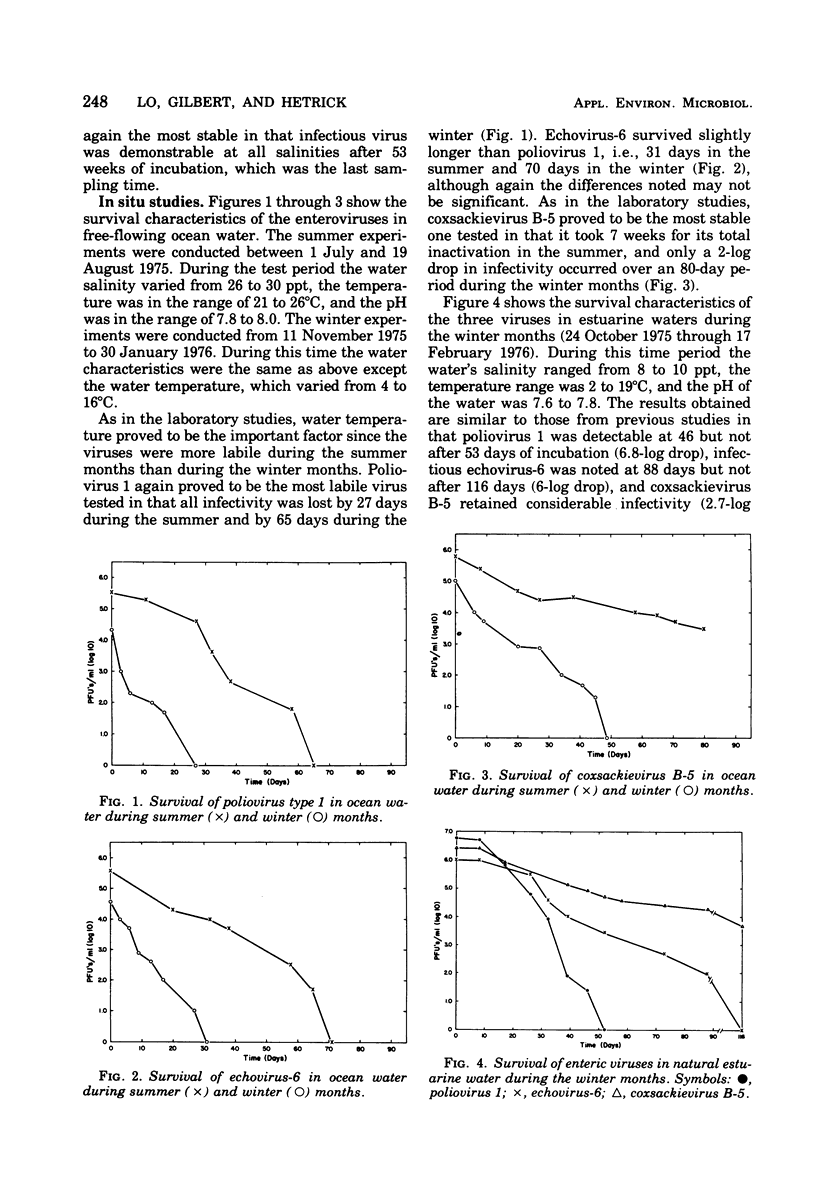
Studies of the effects of temperature and salinity on the survival of three enteric viruses (poliomyelitis type 1, echovirus-6, and coxsackievirus B-5) under controlled laboratory conditions and in situ indicate that temperature rather than salinity is the critical factor affecting their stability, in that the higher the temperature the more rapid was the loss of viral infectivity. In the laboratory studies, all three viruses were quite stable at 4 degrees C, with infectious virus still detectable after 46 weeks of incubation. In situ studies on virus survival in free-flowing estuarine or marine waters showed that, although the viruses were more labile in natural waters than in the laboratory studies, they persisted for several months, in some cases during the winter months. At all temperatures and salinities, coxsackievirus B-5 was the most stable, echovirus-6 was intermediate, and poliovirus 1 was the least stable of the viruses tested.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahling D. R., Berg G., Berman D. BGM, a continuous cell line more sensitive than primary rhesus and African green kidney cells for the recovery of viruses from water. Health Lab Sci. 1974 Oct;11(4):275–282. [PubMed] [Google Scholar]
- De Flora S., De Renzi G. P., Badolati G. Detection of animal viruses in coastal seawater and sediments. Appl Microbiol. 1975 Sep;30(3):472–475. doi: 10.1128/am.30.3.472-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGirolamo R., Liston J., Matches J. R. Survival of virus in chilled, frozen, and processed oysters. Appl Microbiol. 1970 Jul;20(1):58–63. doi: 10.1128/am.20.1.58-63.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu O. C., Seraichekas H. R., Murphy B. L. Viral pollution of shellfish. 1. Some basic facts of uptake. Proc Soc Exp Biol Med. 1966 Nov;123(2):481–487. doi: 10.3181/00379727-123-31520. [DOI] [PubMed] [Google Scholar]
- MASON J. O., McLEAN W. R. Infectious hepatitis traced to the consumption of raw oysters. An epidemiologic study. Am J Hyg. 1962 Jan;75:90–111. doi: 10.1093/oxfordjournals.aje.a120238. [DOI] [PubMed] [Google Scholar]
- METCALF T. G., STILES W. C. THE ACCUMULATION OF ENTERIC VIRUSES BY THE OYSTER, CRASSOSTREA VIRGINICA. J Infect Dis. 1965 Feb;115:68–76. doi: 10.1093/infdis/115.1.68. [DOI] [PubMed] [Google Scholar]
- Mahoney P., Fleischner G., Millman I., London W. T., Blumberg B. S., Arias I. M. Australia antigen: detection and transmission in shellfish. Science. 1974 Jan 11;183(4120):80–81. doi: 10.1126/science.183.4120.80. [DOI] [PubMed] [Google Scholar]
- Metcalf T. G., Stiles W. C. Enteroviruses within an estuarine environment. Am J Epidemiol. 1968 Nov;88(3):379–391. doi: 10.1093/oxfordjournals.aje.a120898. [DOI] [PubMed] [Google Scholar]


