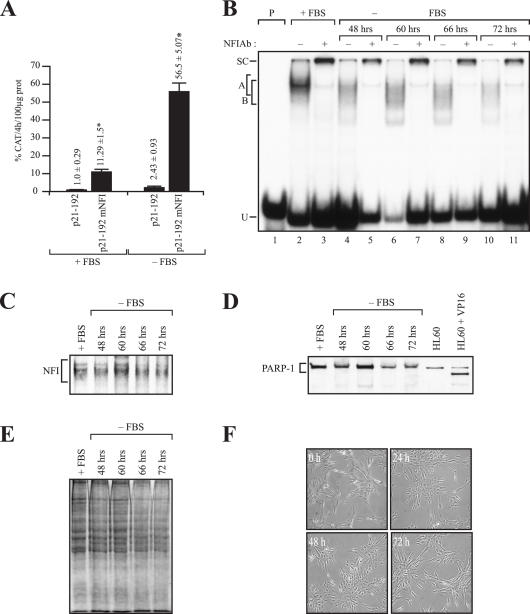
Figure 6.
Influence of serum starvation on NFI binding and p21 promoter function in vitro. (A) The p21–192 construct and its NFI-mutated derivative p21–192 mNFI were transfected into HSF grown either in complete (+FBS) or serum-free DMEM (−FBS) for 72 h. Cells were then harvested and CAT activities determined and normalized to secreted hGH. Asterisks indicate CAT activities that are statistically different from those obtained with the p21–192 construct (P < 0.05; paired samples, t-test). (B) A double-stranded oligonucleotide bearing the high-affinity binding site for human CTF/NFI was incubated with 5 μg proteins from HSFs grown for various periods of time post-transfection (48, 60, 66 and 72 h) in complete (+FBS) or serum-deprived DMEM (−FBS). Formation of DNA–protein complexes was then monitored by EMSA on a 6% native polyacrylamide gel. The position of multiple DNA–protein complexes corresponding to the recognition of the labeled probe by human NFI is indicated (A and B). Extracts from each condition were also incubated either alone (−; lanes 2, 4, 6, 8 and 10) or with 2 μl of the NFI Ab (+; lanes 3, 5, 7, 9 and 11). P: labeled probe with no added protein (lane 1); U: unbound fraction of the labeled probe. (C) Western blot analysis of NFI in nuclear extracts prepared from HSFs cultured in complete DMEM (+FBS) or FBS-free DMEM (−FBS) for 48–72 h. (D) Western blot analysis of PARP-1 expression in HSFs cultured in complete DMEM (+FBS) or FBS-free DMEM (−FBS) for 48–72 h. Nuclear extracts from HL60 cells cultured in the absence and presence of the apoptosis inducer VP16 were also loaded in lanes 6 and 7 as negative and positive controls, respectively. (E) 15 μg nuclear proteins from each of the extracts used above were loaded on a 10% SDS–polyacrylamide gel and stained with Coomassie blue for comparative purpose. (F) Phase-contrast images of HSFs cultured in complete DMEM (+FBS) or FBS-free DMEM (−FBS) for 48–72 h. Magnification, ×200.