Abstract
1. Rabbit left atrial preparations driven electrically at different rates were used for studies on inotropic effects of cations, drugs and coupled pacing. Sino-atrial node-atrial preparations were used for investigating the chronotropic effect of noradrenaline.
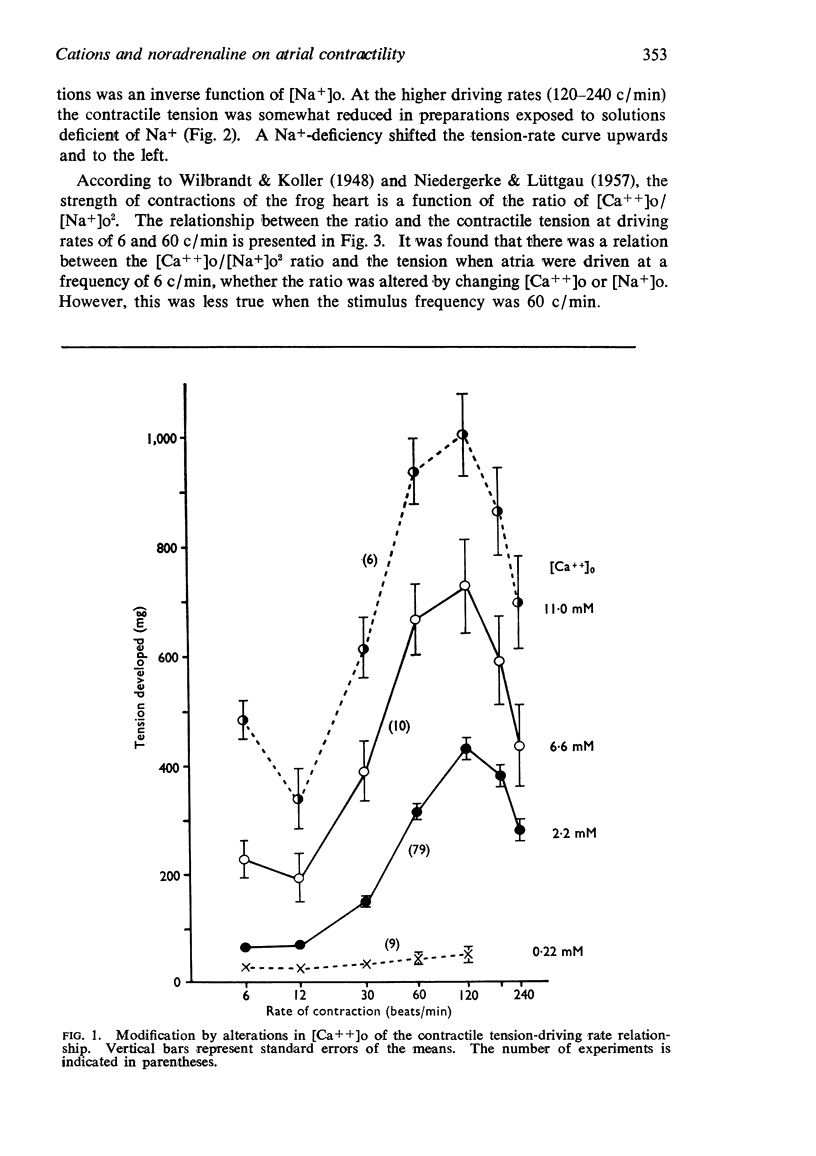
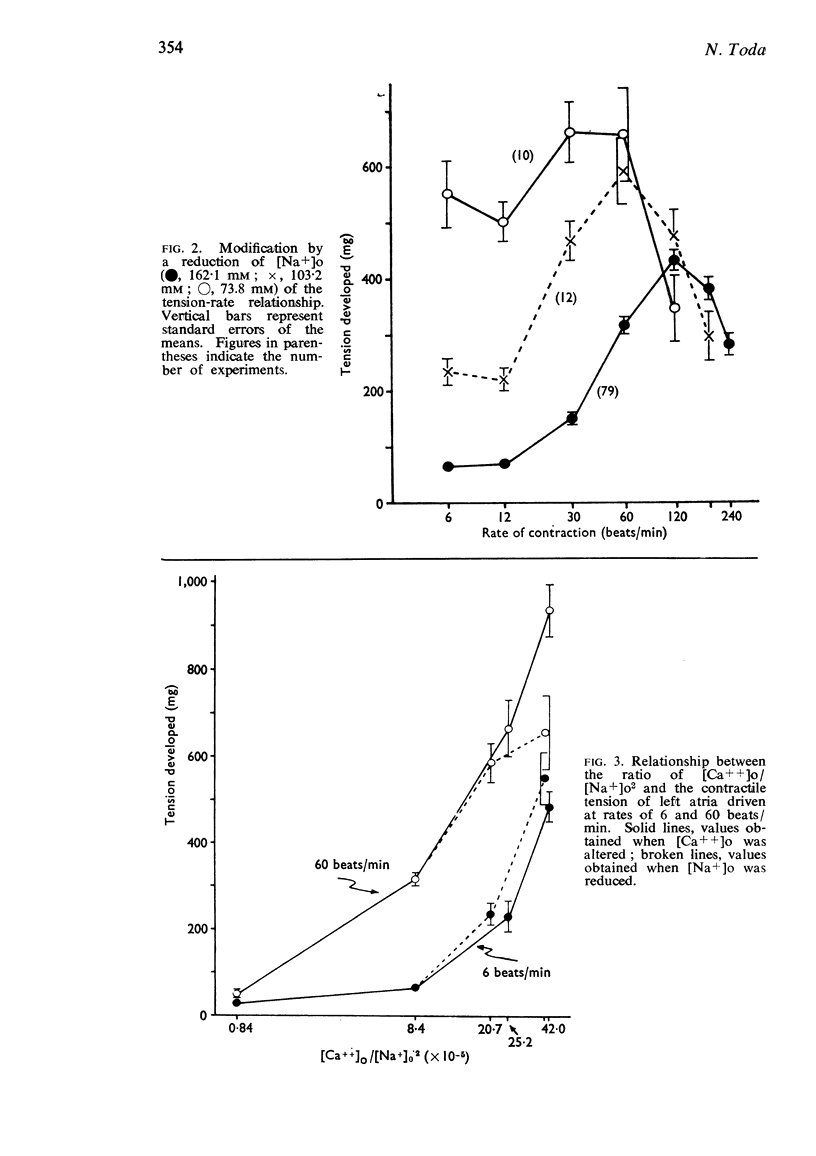
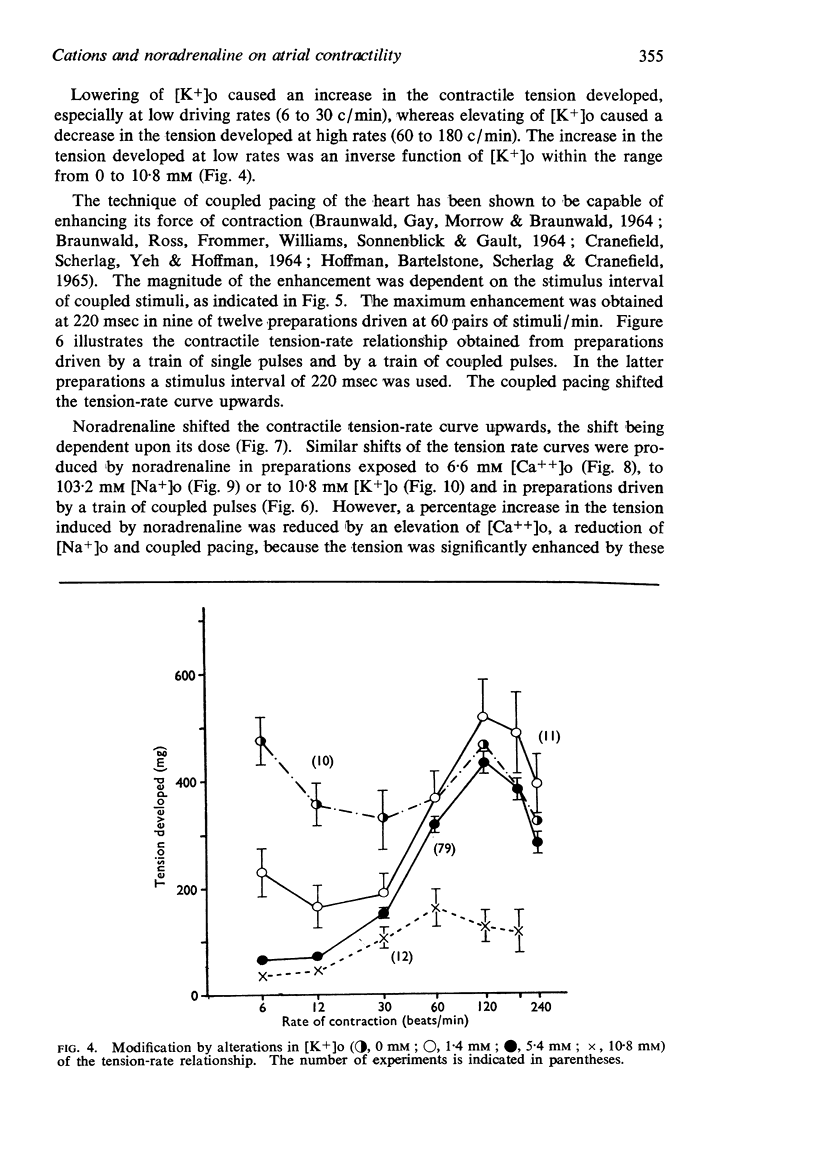
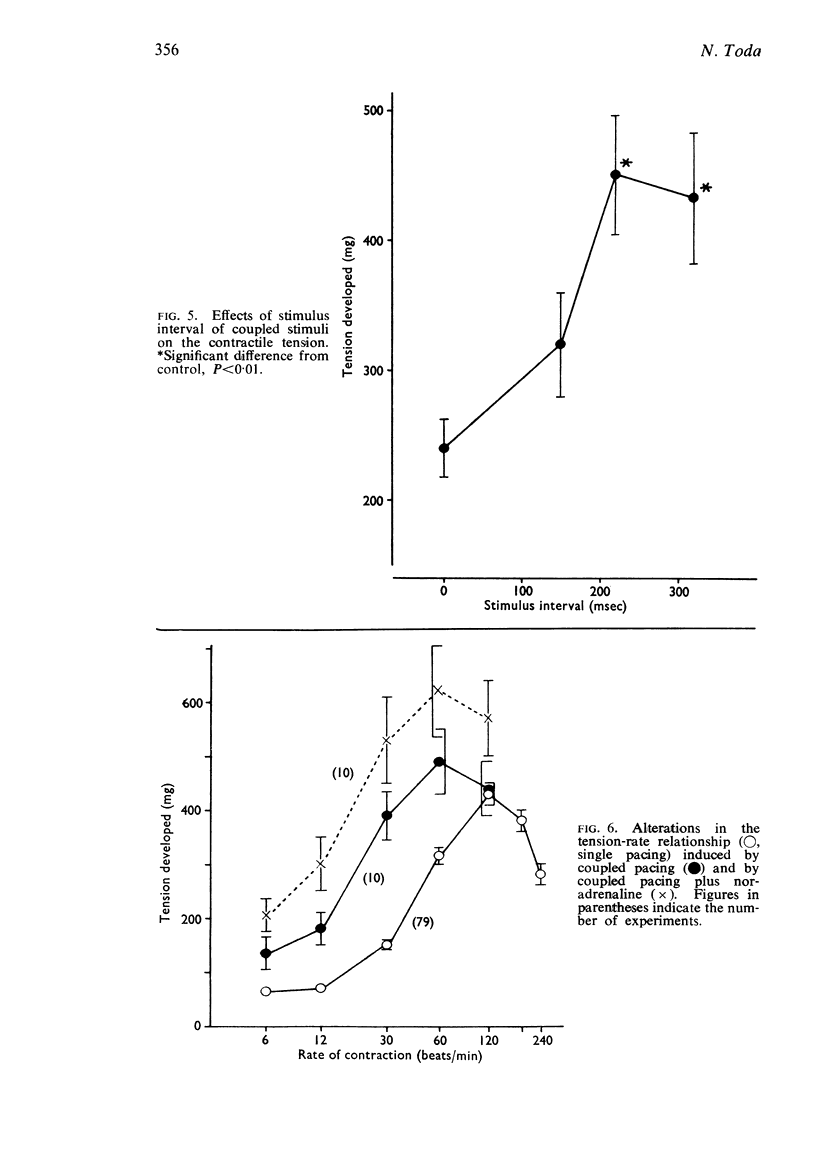
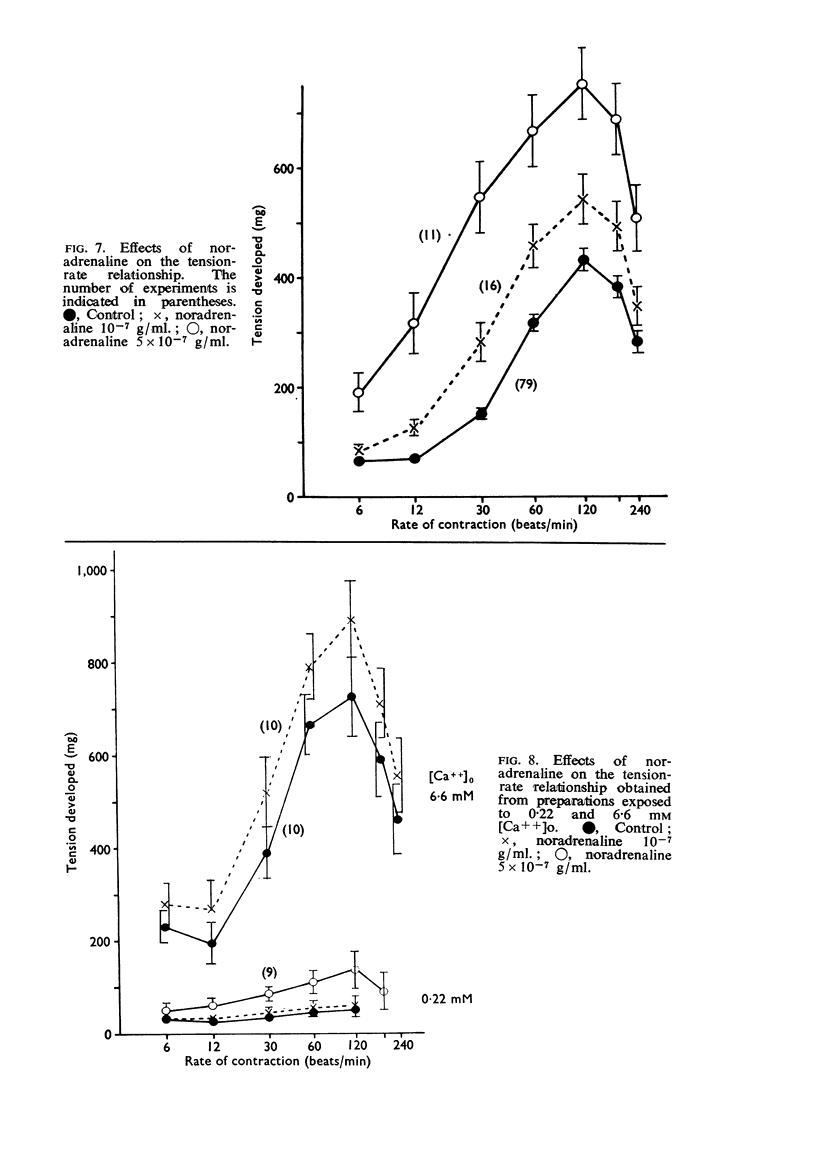
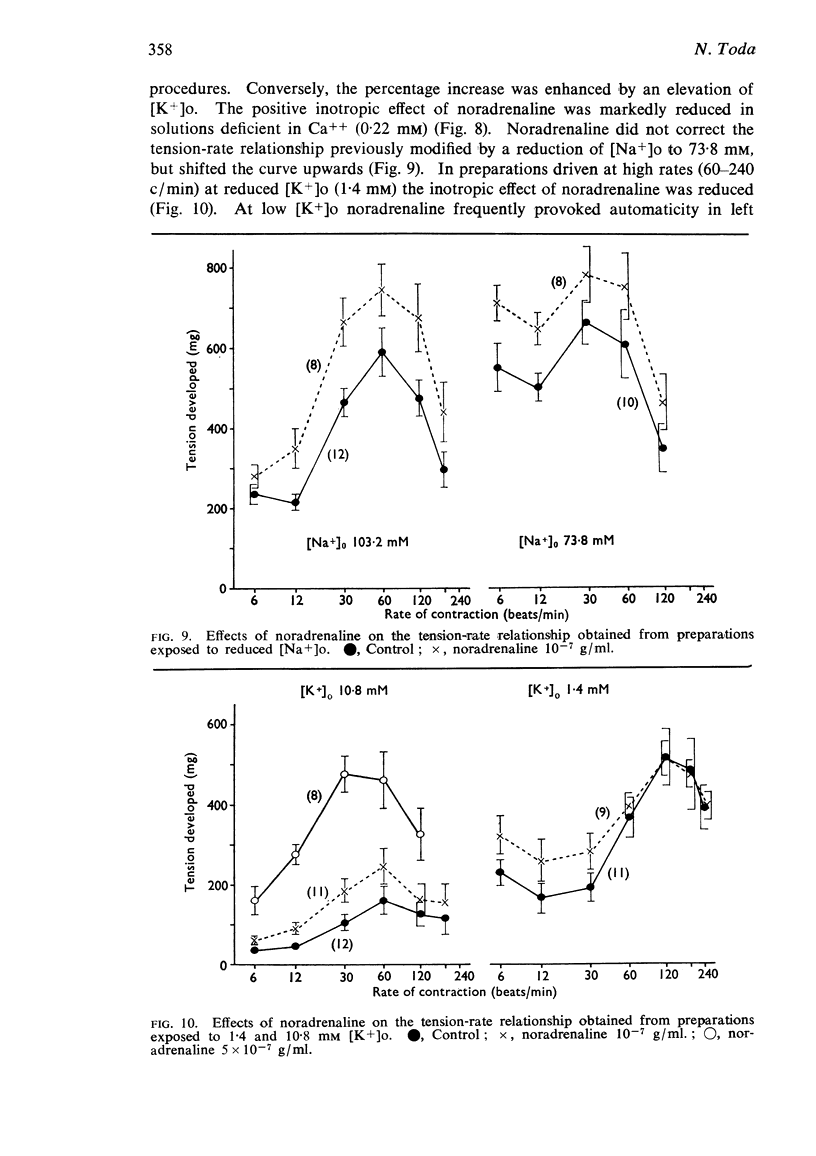
2. The contractile tension-driving rate relationship was moved upwards by an elevation of [Ca++]o, coupled pacing and noradrenaline. In preparations exposed to Na+-poor and K+-free solutions the contractile strength at low driving rates (6 to 30 c/min) was markedly enhanced, but at high rates (120 to 240 c/min) it was not influenced. The contractile strength was reduced at low [Ca++]o and at high [K+]o.
3. The positive inotropic effect of noradrenaline was markedly inhibited by a reduction of [Ca++]o and to some extent by a reduction of [K+]o. The noradrenaline-inotropy was not appreciably affected by an elevation of [Ca++]o and a reduction of [Na+]o.
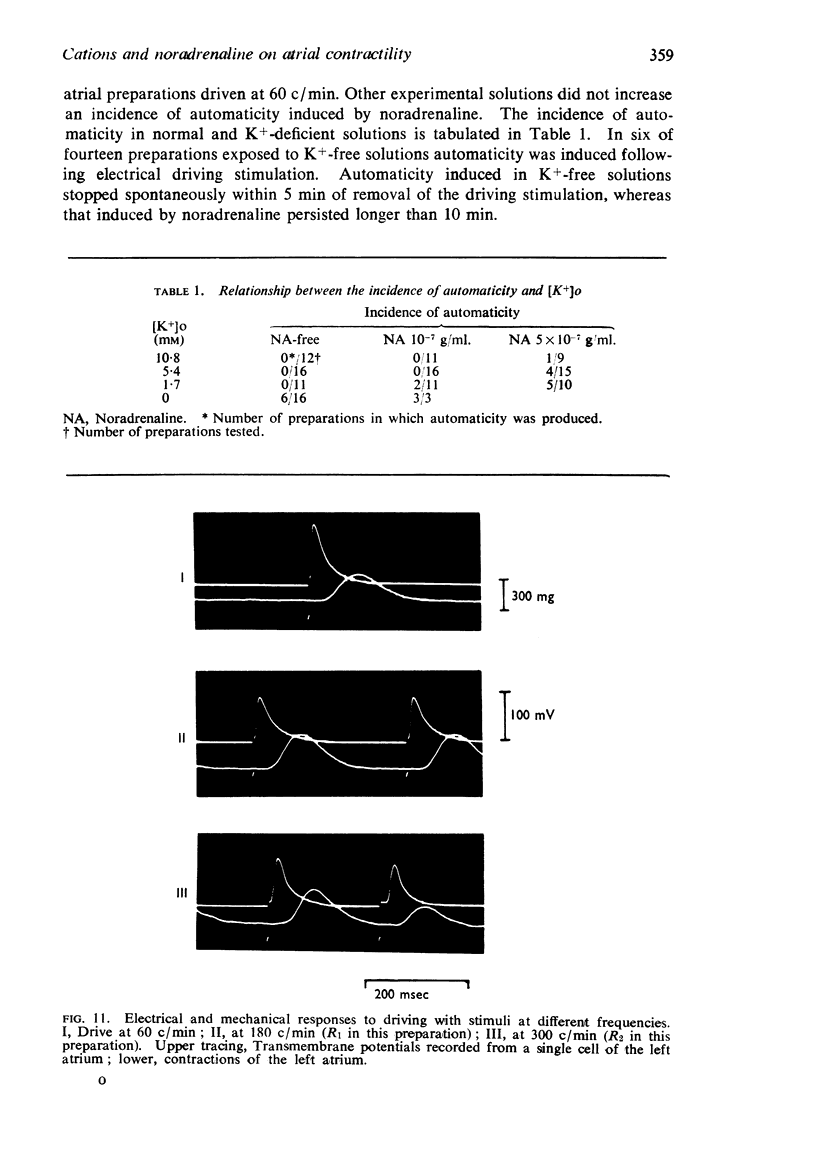
4. Cardiac excitability studied in preparations driven at high rates was enhanced by noradrenaline, a reduction of [K+]o and an elevation of [Ca++]o, but was reduced at low [Ca++]o, low [Na+]o and high [K+]o.
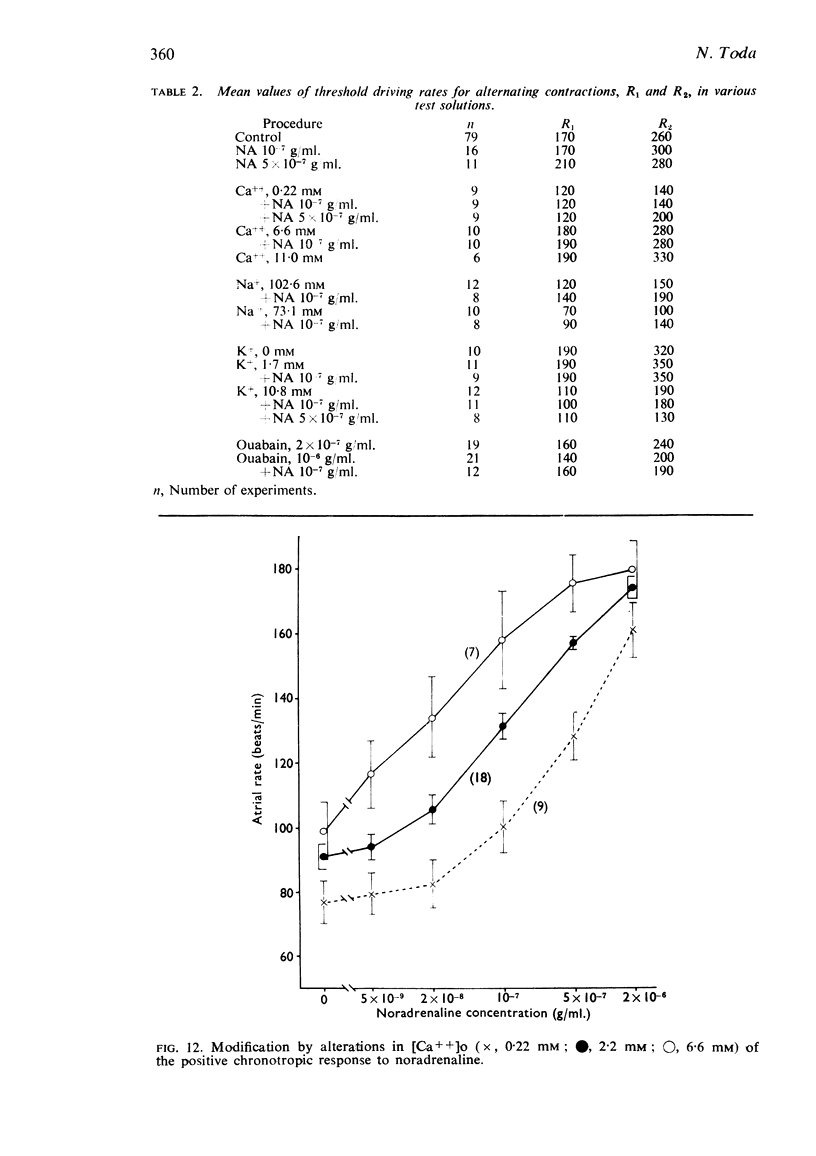
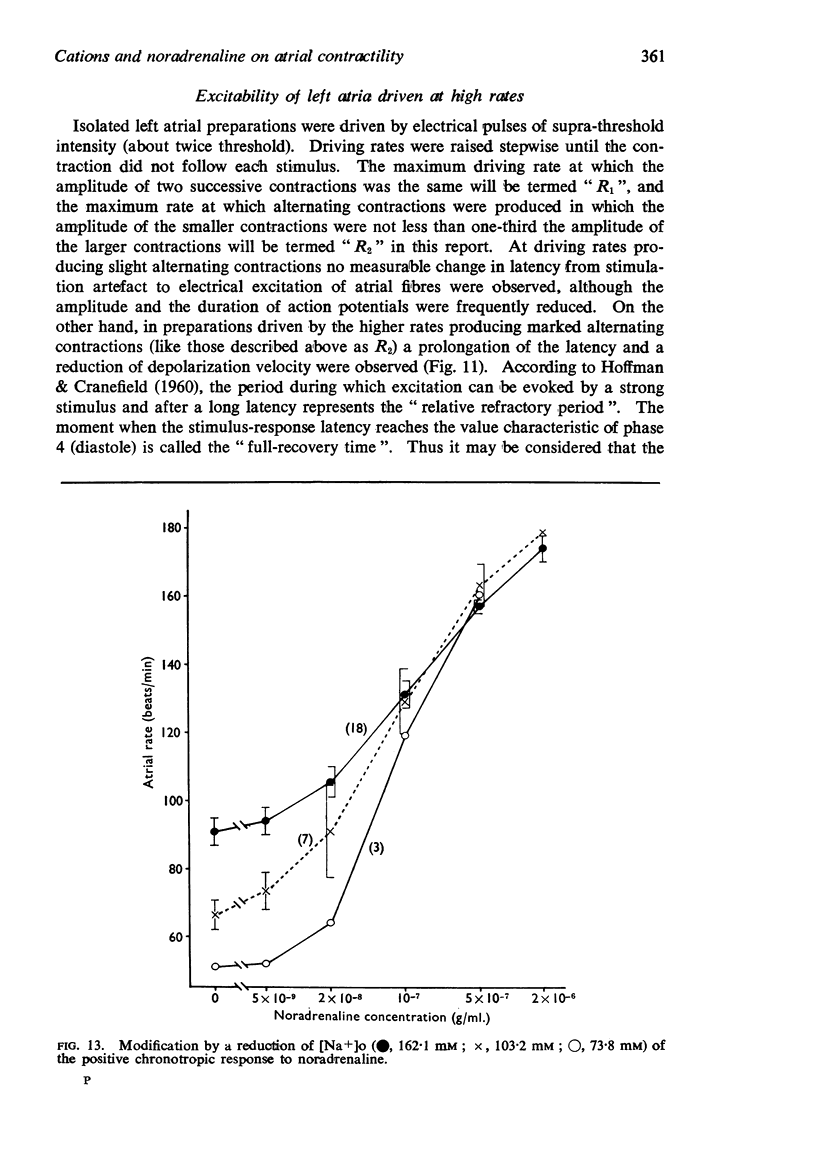
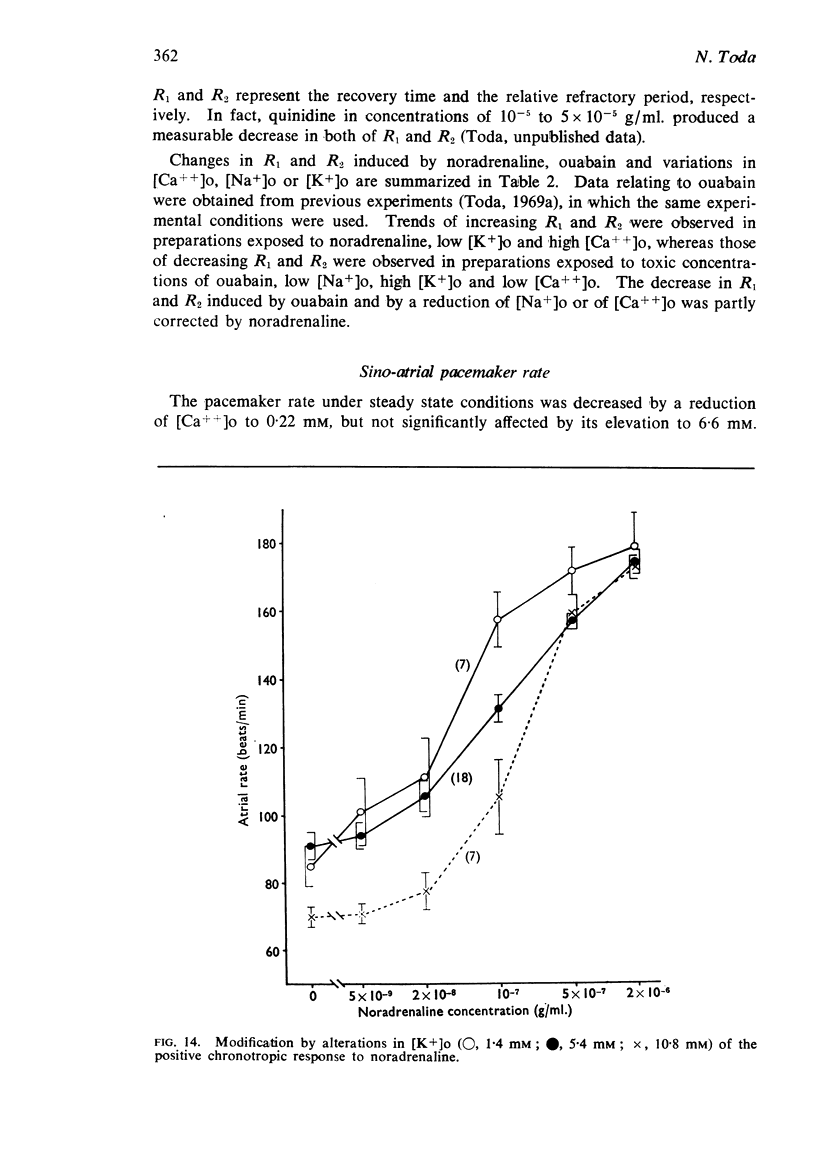
5. The positive chronotropic response to noradrenaline was enhanced at high [Ca++]o, low [Na+]o and low [K+]o, but was reduced in solutions deficient in Ca++ or rich in K+.
6. Inotropic effects of the ions and of coupled pacing were compared with those of ouabain. It is suggested that characteristic changes in the tension-rate curve seen in Na+-poor, K+-free and ouabain-containing solutions are correlated with an inhibition of active processes in the cardiac cell membrane, which affect ionic movements across it. It seems likely that mechanisms mediating adrenergic responses of the contractile tissue and the S-A node are associated with [Ca++]o.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER W. W., BAKER J. M. Effects of epinephrine and calcium on the electrogram of the ouabainized frog heart. Circ Res. 1955 May;3(3):274–279. doi: 10.1161/01.res.3.3.274. [DOI] [PubMed] [Google Scholar]
- BRAUNWALD E., ROSS J., Jr, FROMMER P. L., WILLIAMS J. F., Jr, SONNENBLICK E. H., GAULT J. H. CLINICAL OBSERVATIONS ON PAIRED ELECTRICAL STIMULATION OF THE HEART. EFFECTS ON VENTRICULAR PERFORMANCE AND HEART RATE. Am J Med. 1964 Nov;37:700–711. doi: 10.1016/0002-9343(64)90019-1. [DOI] [PubMed] [Google Scholar]
- BRAUNWALD N. S., GAY W. A., Jr, MORROW A. G., BRAUNWALD E. SUSTAINED, PAIRED ELECTRICAL STIMULI: SLOWING OF THE VENTRICULAR RATE AND AUGMENTATION OF CONTRACTILE FORCE. Am J Cardiol. 1964 Sep;14:385–393. doi: 10.1016/0002-9149(64)90083-9. [DOI] [PubMed] [Google Scholar]
- Badeer H. S., Ryo U. Y., Gassner W. F., Kass E. J., Cavaluzzi J., Gilbert J. L., Brooks C. M. Factors affecting pulsus alternans in the rapidly driven heart and papillary muscle. Am J Physiol. 1967 Nov;213(5):1095–1101. doi: 10.1152/ajplegacy.1967.213.5.1095. [DOI] [PubMed] [Google Scholar]
- CRANEFIELD P. F., SCHERLAG B. J., YEH B. K., HOFFMAN B. F. TREATMENT OF ACUTE CARDIAC FAILURE BY MAINTAINED POSTEXTRASYSTOLIC POTENTIATION. Bull N Y Acad Med. 1964 Nov;40:903–913. [PMC free article] [PubMed] [Google Scholar]
- Dhalla N. S., Braxton A. Influence of some inhibitors and ions on the positive inotropic action of epinephrine, tyramine and calcium. J Pharmacol Exp Ther. 1968 Jun;161(2):238–246. [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- GOVIER W. C., HOLLAND W. C. EFFECTS OF OUABAIN ON TISSUE CALCIUM AND CALCIUM EXCHANGE IN PACEMAKER OF TURTLE HEART. Am J Physiol. 1964 Jul;207:195–198. doi: 10.1152/ajplegacy.1964.207.1.195. [DOI] [PubMed] [Google Scholar]
- GROSSMAN A., FURCHGOTT R. F. THE EFFECTS OF EXTERNAL CALCIUM CONCENTRATION ON THE DISTRIBUTION AND EXCHANGE OF CALCIUM IN RESTING AND BEATING GUINEA-PIG AURICLES. J Pharmacol Exp Ther. 1964 Jan;143:107–119. [PubMed] [Google Scholar]
- GROSSMAN A., FURCHGOTT R. F. THE EFFECTS OF VARIOUS DRUGS ON CALCIUM EXCHANGE IN THE ISOLATED GUINEA-PIG LEFT AURICLE. J Pharmacol Exp Ther. 1964 Aug;145:162–172. [PubMed] [Google Scholar]
- Gousios A. G., Felts J. M., Havel R. J. Effects of ouabain on force of contraction, oxygen consumption, and metabolism of free fatty acids in the perfused rabbit heart. Circ Res. 1967 Oct;21(4):445–448. doi: 10.1161/01.res.21.4.445. [DOI] [PubMed] [Google Scholar]
- HAJDU S., LEONARD E. The cellular basis of cardiac glycoside action. Pharmacol Rev. 1959 Jun;11(2 Pt 1):173–209. [PubMed] [Google Scholar]
- HOFFMAN B. F., BARTELSTONE H. J., SCHERLAG B. J., CRANEFIELD P. F. EFFECTS OF POSTEXTRASYSTOLIC POTENTIATION ON NORMAL AND FAILING HEARTS. Bull N Y Acad Med. 1965 May;41:498–534. [PMC free article] [PubMed] [Google Scholar]
- Horn R. S., Aronson C. E., Hess M. E., Haugaard N. The effect of metabolic inhibitors on the response of the perfused rat heart to epinephrine. Biochem Pharmacol. 1967 Nov;16(11):2109–2116. doi: 10.1016/0006-2952(67)90008-1. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Davies R. E. The effect of 2,4-dinitrofluorobenzene on the activity of striated muscle. J Biol Chem. 1965 Oct;240(10):3996–4001. [PubMed] [Google Scholar]
- Iversen L. L., Kravitz E. A. Sodium dependence of transmitter uptake at adrenergic nerve terminals. Mol Pharmacol. 1966 Jul;2(4):360–362. [PubMed] [Google Scholar]
- KAVALER F., FISHER V. J., STUCKEY J. H. THE POTENTIATED CONTRACTION AND VENTRICULAR "CONTRACTILITY". Bull N Y Acad Med. 1965 Jun;41:592–601. [PMC free article] [PubMed] [Google Scholar]
- KLAUS W., KUSCHINSKY G. [On the effect of digitoxigenin on the cellular calcium exchange in heart muscle tissue]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1962;244:237–253. [PubMed] [Google Scholar]
- KOCH-WESER J., BLINKS J. R. THE INFLUENCE OF THE INTERVAL BETWEEN BEATS ON MYOCARDIAL CONTRACTILITY. Pharmacol Rev. 1963 Sep;15:601–652. [PubMed] [Google Scholar]
- LEE K. S., YU D. H. A STUDY OF THE SODIUM- AND POTASSIUM-ACTIVATED ADENOSINETRIPHOSPHATASE ACTIVITY OF HEART MICROSOMAL FRACTION. Biochem Pharmacol. 1963 Nov;12:1253–1264. [PubMed] [Google Scholar]
- LULLMANN H., HOLLAND W. Influence of ouabain on an exchangeable calcium fraction, contractile force, and resting tension of guinea-pig atria. J Pharmacol Exp Ther. 1962 Aug;137:186–192. [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSFIELD P. B., MCDONALD R. H., Jr SOME METABOLIC ASPECTS OF PAIRED PACING OF THE HEART. Bull N Y Acad Med. 1965 Jun;41:700–711. [PMC free article] [PubMed] [Google Scholar]
- MUELLER P. OUABAIN EFFECTS ON CARDIAC CONTRACTION, ACTION POTENTIAL, AND CELLULAR POTASSIUM. Circ Res. 1965 Jul;17:46–56. doi: 10.1161/01.res.17.1.46. [DOI] [PubMed] [Google Scholar]
- NIEDERGERKE R., HARRIS E. J. Accumulation of calcium (or strontium) under conditions of increasing contractility. Nature. 1957 May 25;179(4569):1068–1069. doi: 10.1038/1791068a0. [DOI] [PubMed] [Google Scholar]
- NIEDERGERKE R., LUTTGAU H. C. Antagonism between calcium and sodium ions. Nature. 1957 May 25;179(4569):1066–1067. doi: 10.1038/1791066a0. [DOI] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dual effect of calcium on the action potential of the frog's heart. J Physiol. 1966 May;184(2):291–311. doi: 10.1113/jphysiol.1966.sp007916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand R. K. Facilitation of heart muscle contraction and its dependence on external calcium and sodium. J Physiol. 1968 May;196(2):311–325. doi: 10.1113/jphysiol.1968.sp008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE E., GOERKE R. J., STORM S. R. CAT HEART MUSCLE IN VITRO. IV. INHIBITION OF TRANSPORT IN QUIESCENT MUSCLES. J Gen Physiol. 1964 Jan;47:531–543. doi: 10.1085/jgp.47.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS J., Jr, SONNENBLICK E. H., KAISER G. A., FROMMER P. L., BRAUNWALD E. ELECTROAUGMENTATION OF VENTRICULAR PERFORMANCE AND OXYGEN CONSUMPTION BY REPETITIVE APPLICATION OF PAIRED ELECTRICAL STIMULI. Circ Res. 1965 Apr;16:332–342. doi: 10.1161/01.res.16.4.332. [DOI] [PubMed] [Google Scholar]
- Reiter M. Der Einfluss der Natriumionen auf die Beziehung zwischen Frequenz und Kraft der Kontraktion des isolierten Meerschweinchenmyokards. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;254(3):261–286. [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol. 1967 Sep;192(2):479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKUL A. A., HOLLAND W. C. Effects of ouabain on Ca45 entry in quiescent and electrically driven rabbit atria. Am J Physiol. 1960 Sep;199:457–459. doi: 10.1152/ajplegacy.1960.199.3.457. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. ATP-dependent Ca++-extrusion from human red cells. Experientia. 1966 Jun 15;22(6):364–365. doi: 10.1007/BF01901136. [DOI] [PubMed] [Google Scholar]
- Shelburne J. C., Serena S. D., Langer G. A. Rate-tension staircase in rabbit ventricular muscle: relation to ionic exchange. Am J Physiol. 1967 Nov;213(5):1115–1124. doi: 10.1152/ajplegacy.1967.213.5.1115. [DOI] [PubMed] [Google Scholar]
- Teiger D., Scheider F., Farah A. The effects of sodium ion and rate of stimulation on the refractory period of isolated rabbit atrial muscle. J Pharmacol Exp Ther. 1967 Jan;155(1):58–68. [PubMed] [Google Scholar]
- Toda N. Influence of sodium ions on the membrane potential of the sino-atrial node in response to sympathetic nerve stimulation. J Physiol. 1968 Jun;196(3):677–691. doi: 10.1113/jphysiol.1968.sp008529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N., West T. C. Modification by sodium and calcium of the cardiotoxicity induced by ouabain. J Pharmacol Exp Ther. 1966 Nov;154(2):239–249. [PubMed] [Google Scholar]
- Toda N., West T. C. The influence of ouabain on cholinergic responses in the sinoatrial node. J Pharmacol Exp Ther. 1966 Jul;153(1):104–113. [PubMed] [Google Scholar]
- WINEGRAD S., SHANES A. M. Calcium flux and contractility in guinea pig atria. J Gen Physiol. 1962 Jan;45:371–394. doi: 10.1085/jgp.45.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]


