Abstract
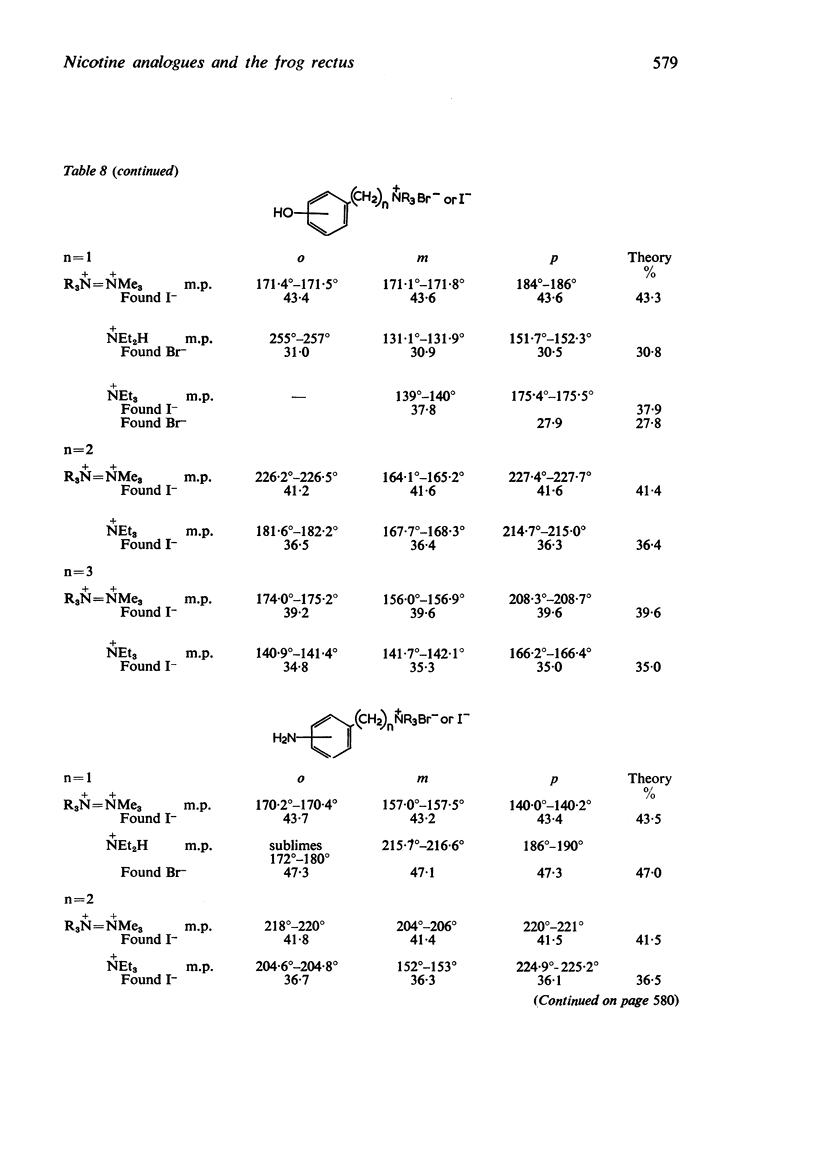
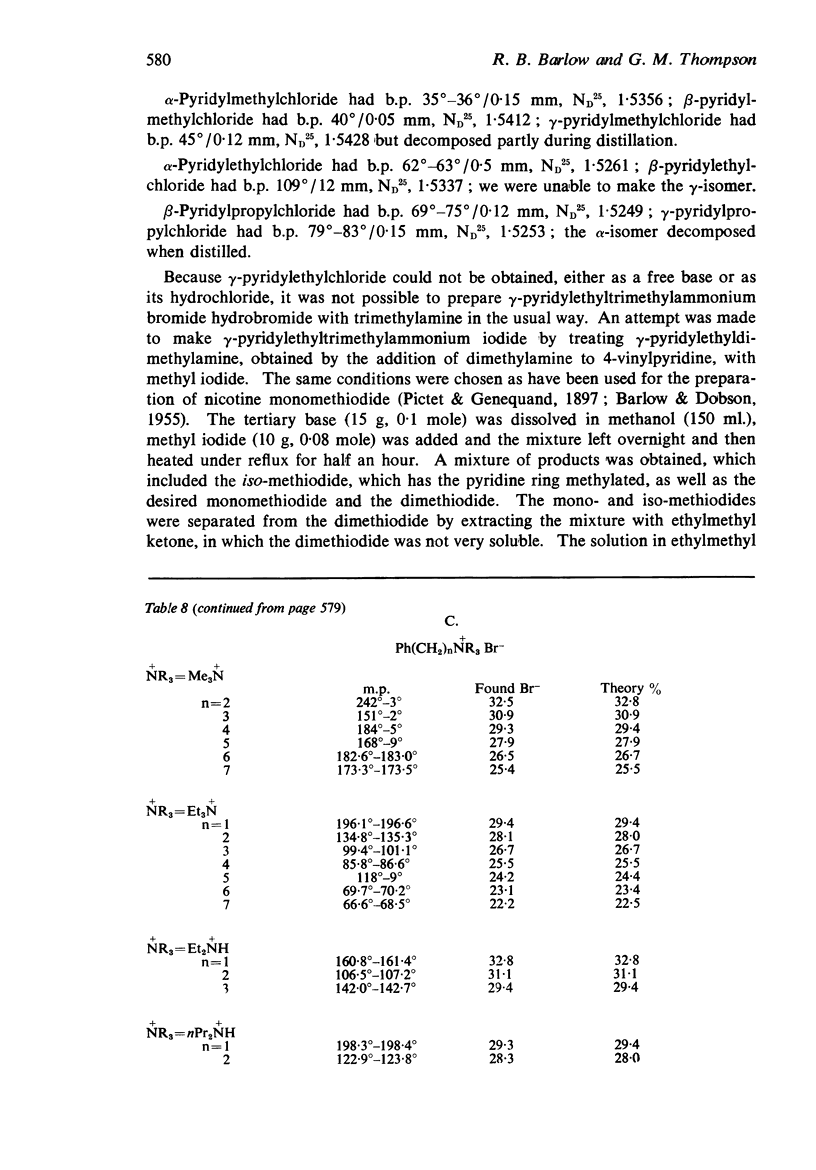
1. Series of pyridylalkyl- and substituted phenylalkyl-trimethylammonium salts, triethylammonium salts, diethylamines and di-n-propylamines have been made. The substituents in the benzene ring were nitro, chloro, bromo, methoxy, hydroxy and amino groups and the alkyl residues had one, two, or three methylene groups separating the aromatic nucleus from the cationic head.
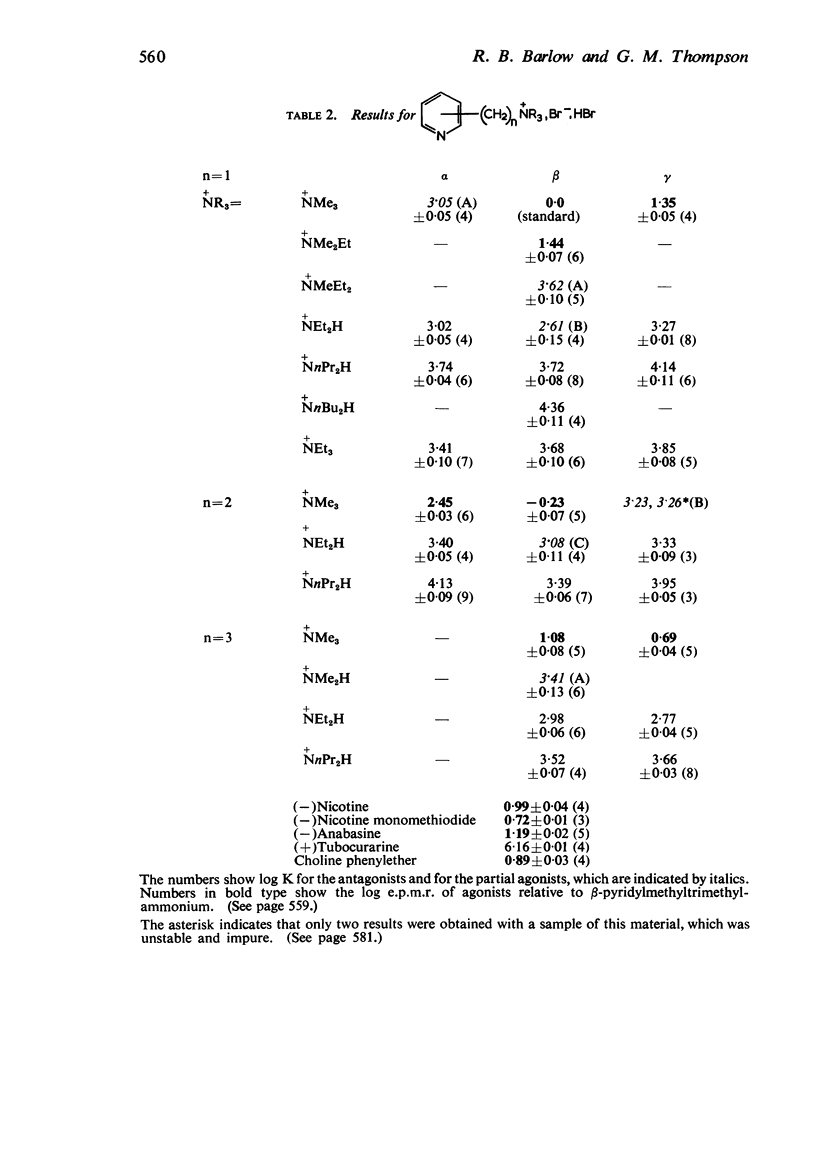
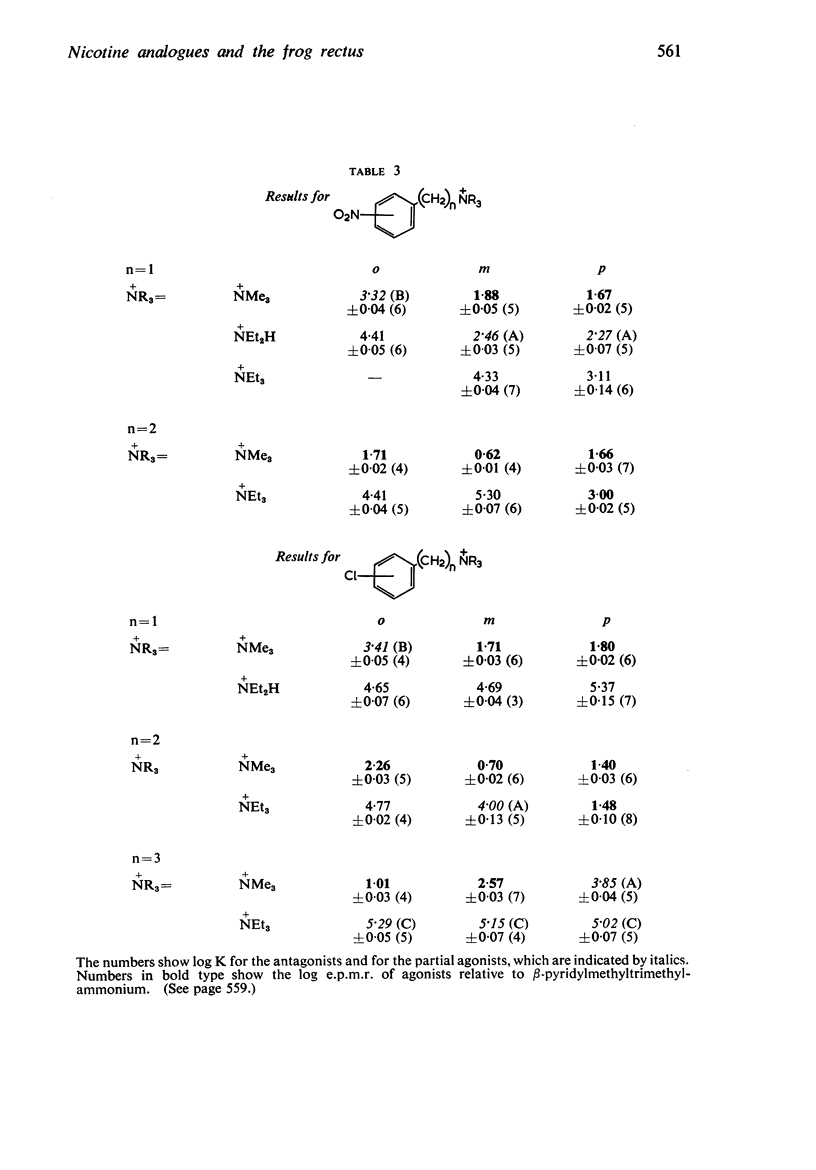
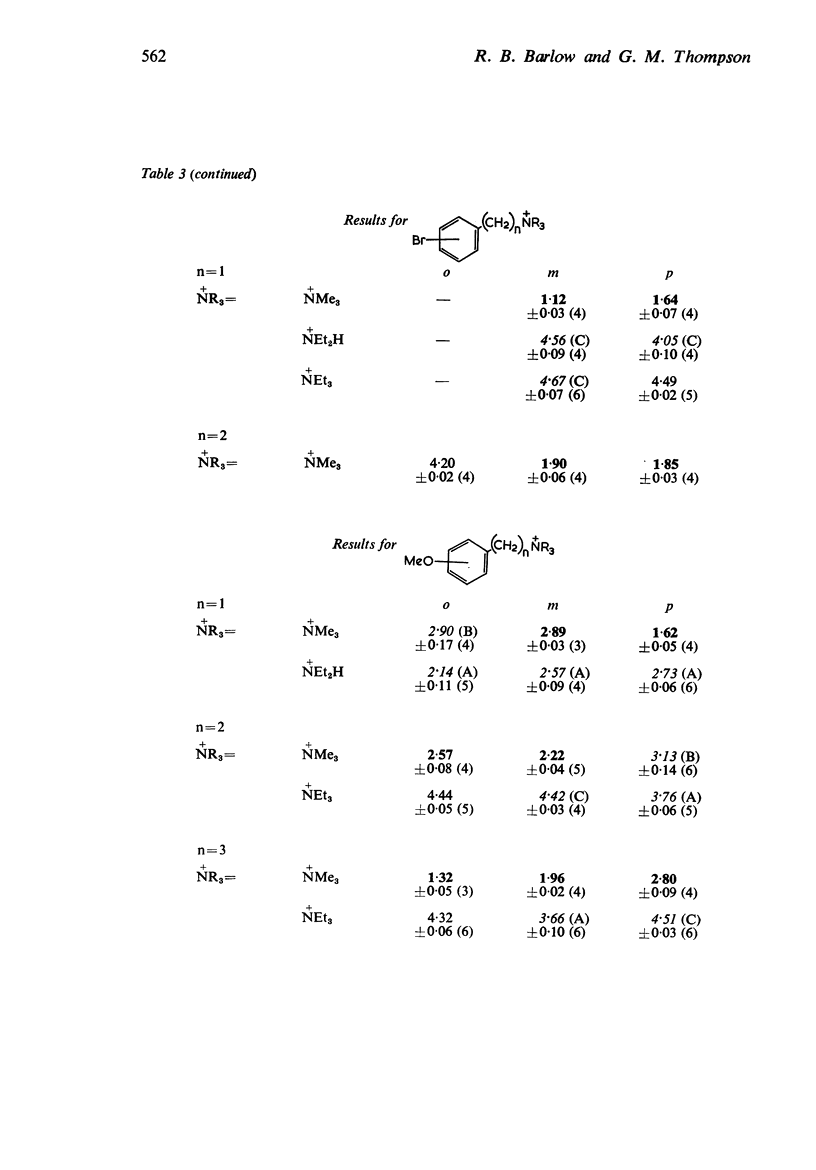
2. Most of the trimethylammonium compounds caused a contracture of the frog rectus muscle, but some were partial agonists and a few were antagonists. The di-n-propylamines were all antagonists, as were most of the diethylamines and triethylammonium compounds, though some of these were partial agonists and a few triethylammonium compounds were agonists. The affinities of the antagonists and partial agonists for the receptors stimulated by β-pyridylmethyltrimethylammonium (and by nicotine) were measured. The equipotent molar ratios of all the agonists were measured relative to β-pyridylmethyltrimethylammonium.
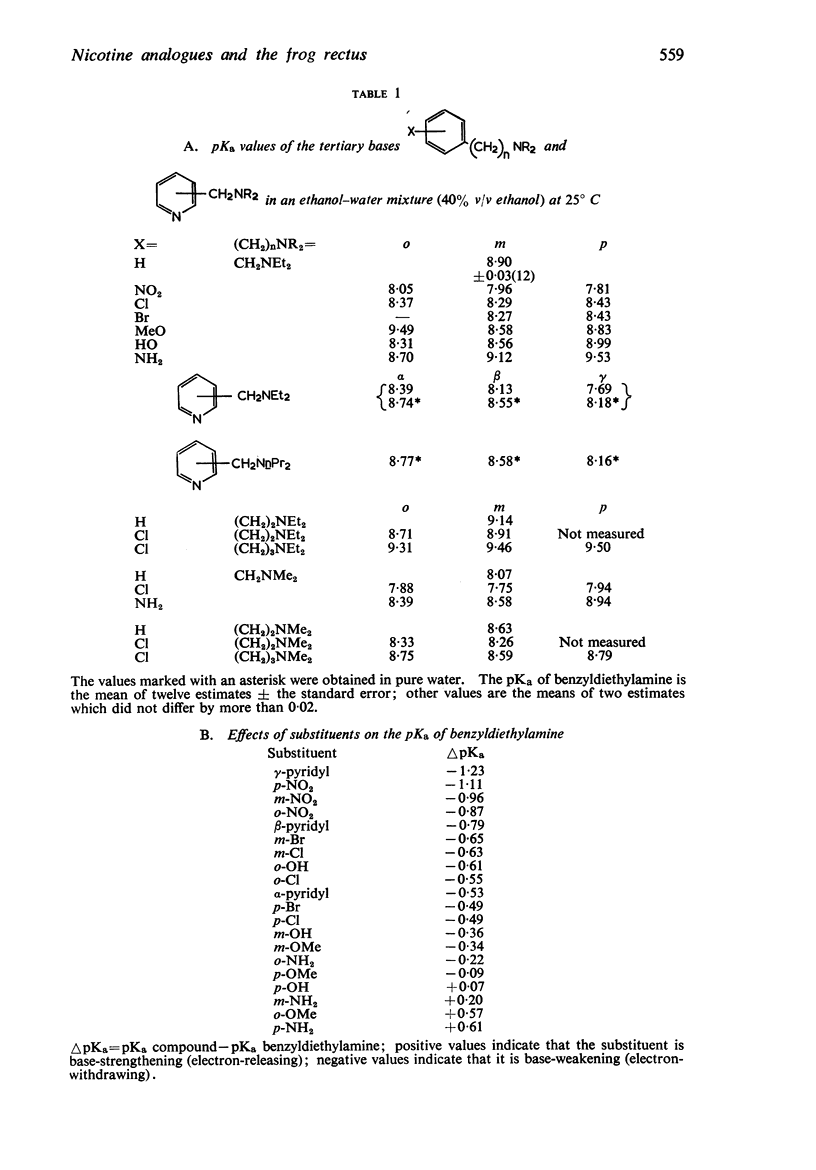
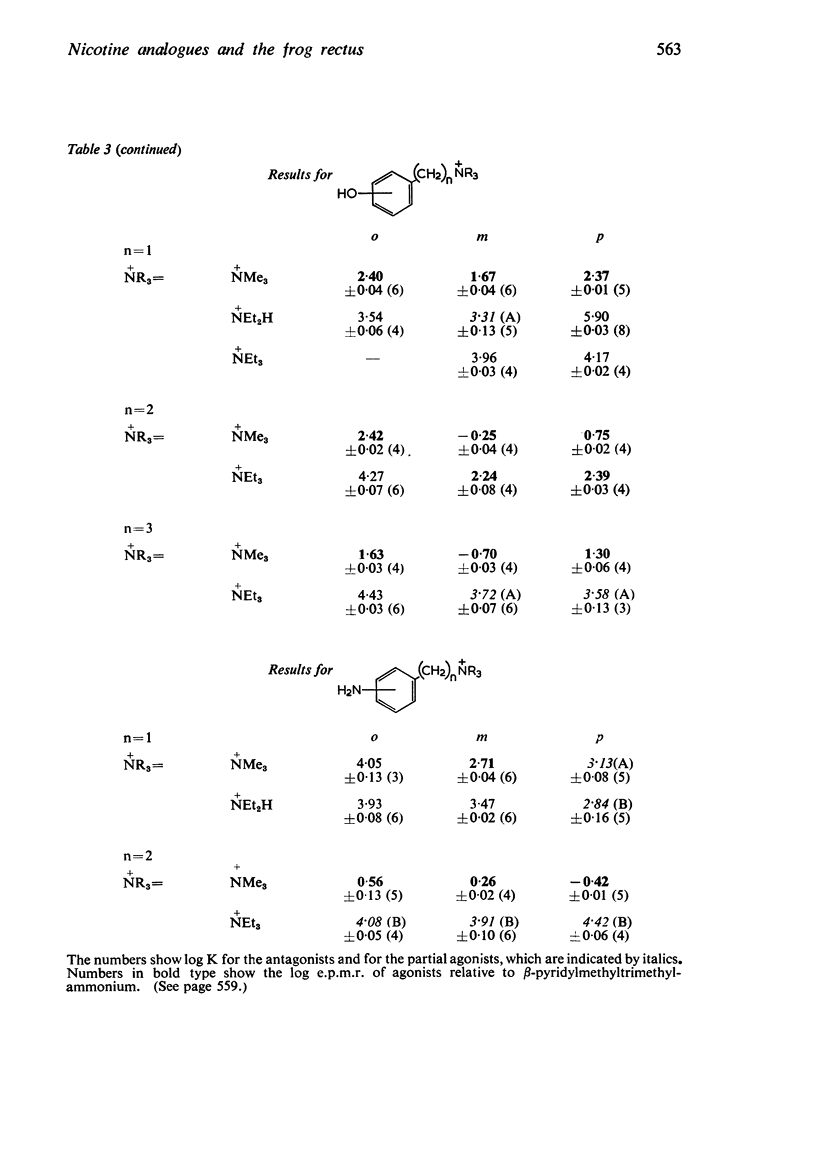
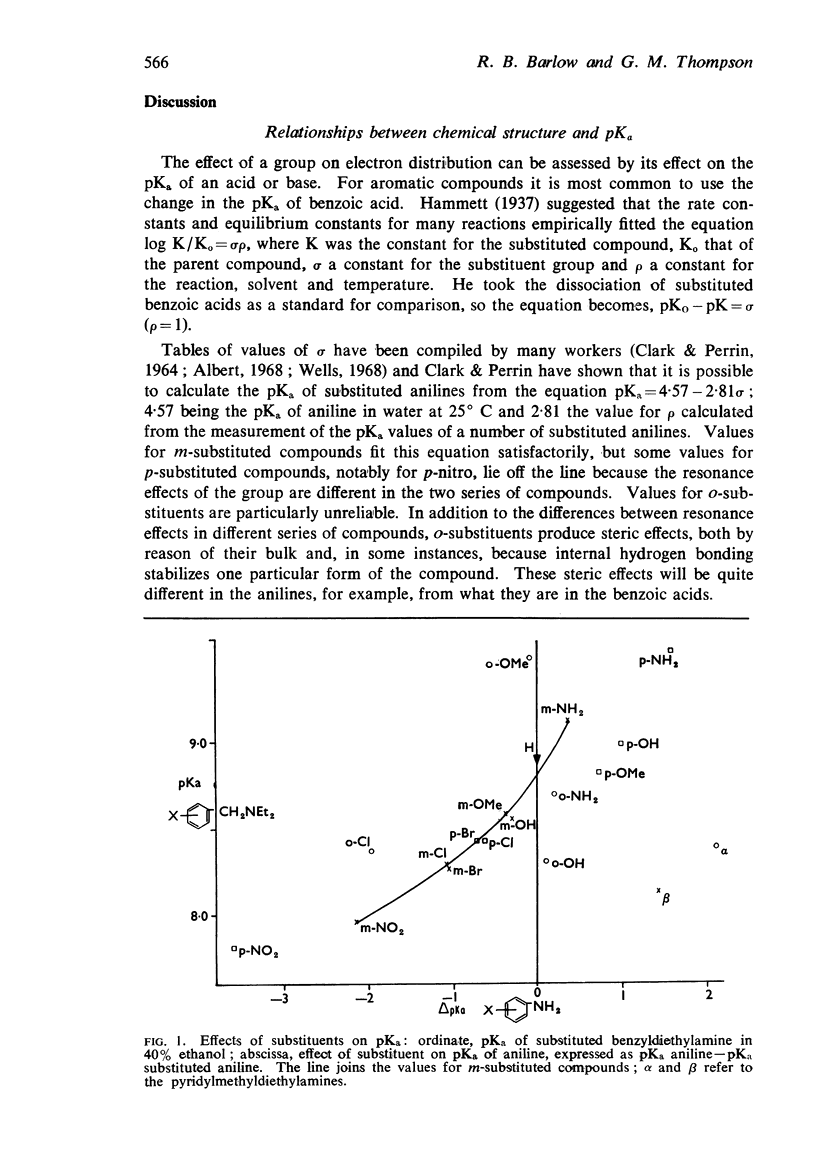
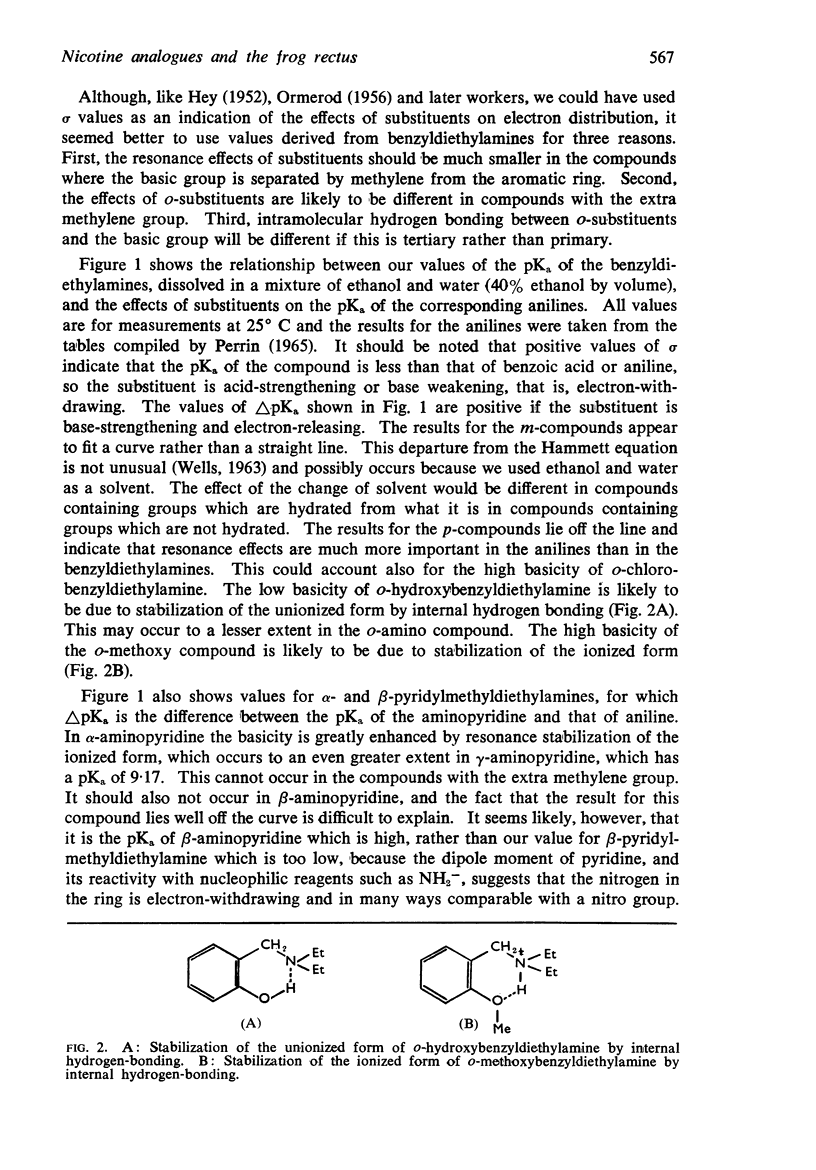
3. The dissociation constants of the pyridylmethyldiethylamines and substituted benzyldiethylamines were measured. The effects of substituents on the pKa of benzyldiethylamine were similar to their effects on the pKa of aniline, though there were differences with some of the o-substituted compounds, which could be attributed to internal hydrogen-bond formation.
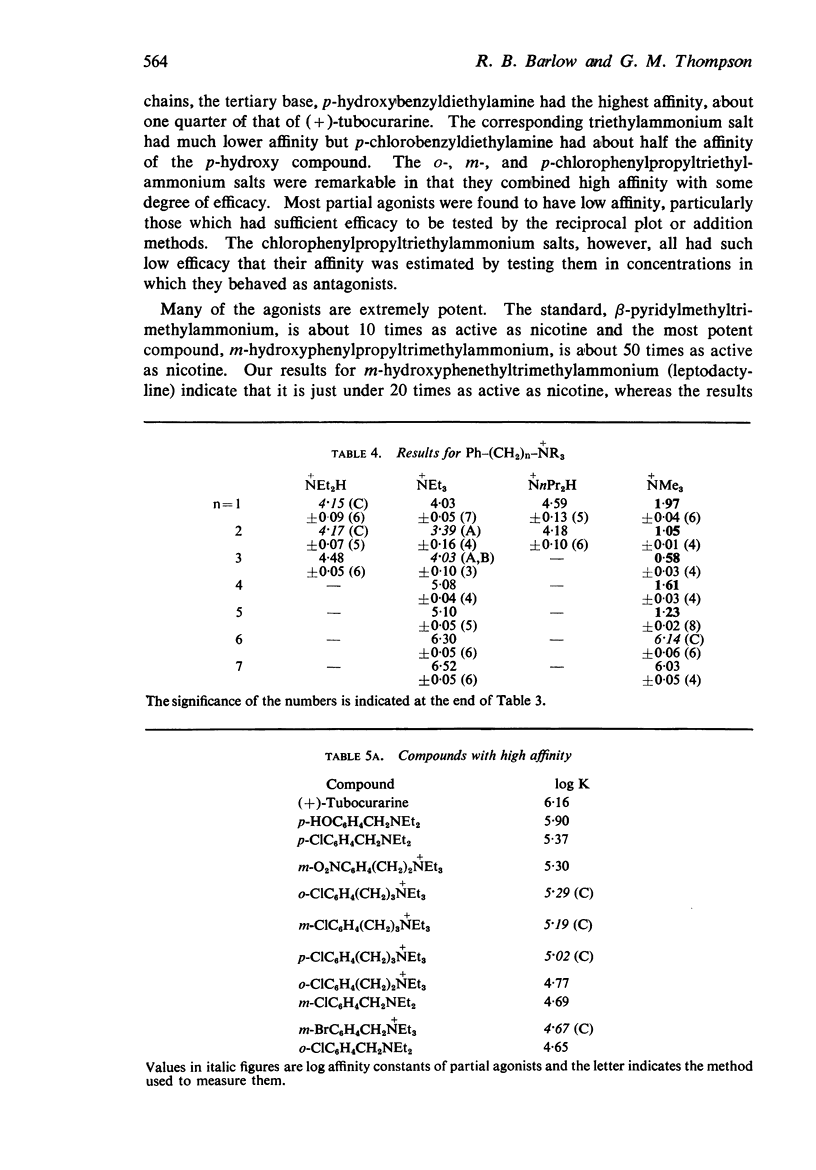
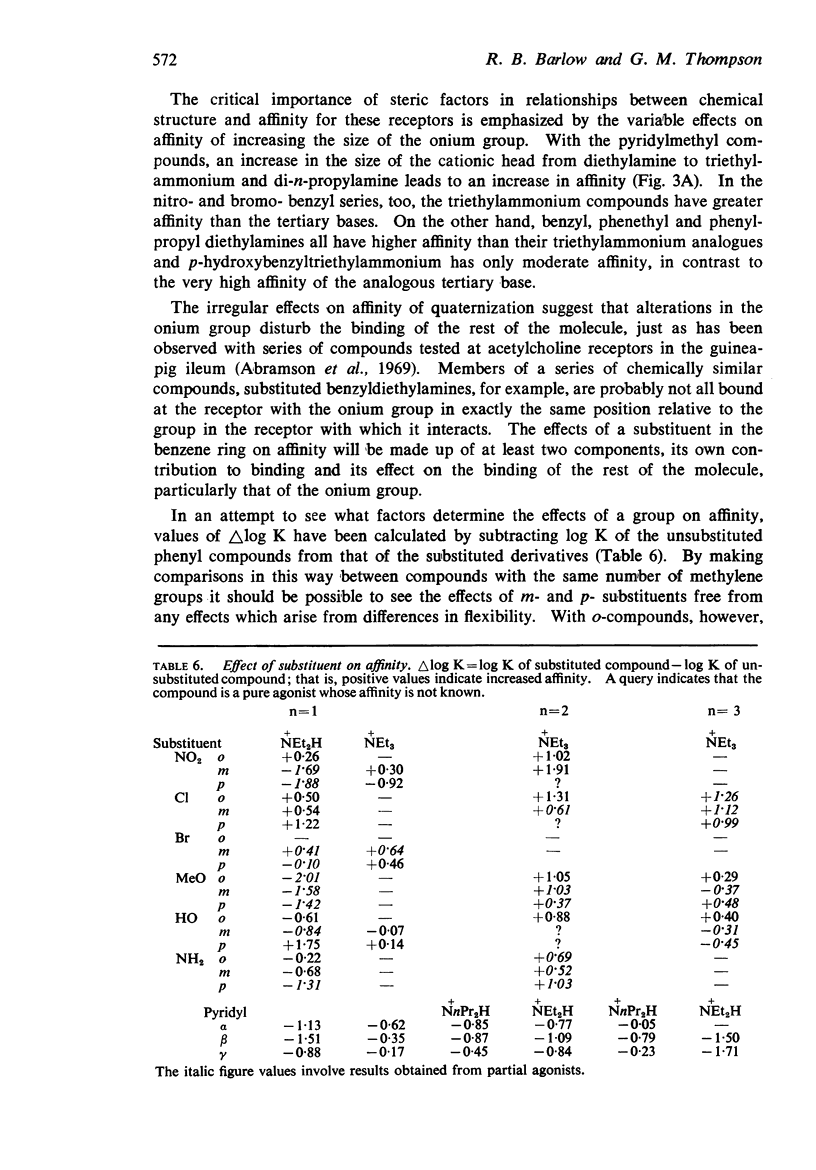
4. There is no obvious correlation between the effects of a substituent on the pKa of benzyldiethylamine and its effects on affinity. Although increasing the size of the cationic group usually increased affinity, it did not always do so. The compounds with the highest affinity, p-hydroxybenzyldiethylamine (log K, 5·90) had about half the affinity of (+)-tubocurarine (log K, 6·11), but the triethylammonium analogue (log K, 4·17) had only about one-fiftieth of the affinity of the tertiary base. The binding of the drug to the receptor appears to involve many factors which include the size of the groups as well as their electron-releasing or withdrawing nature and other properties, such as their polar and lipophilic or lipophobic character.
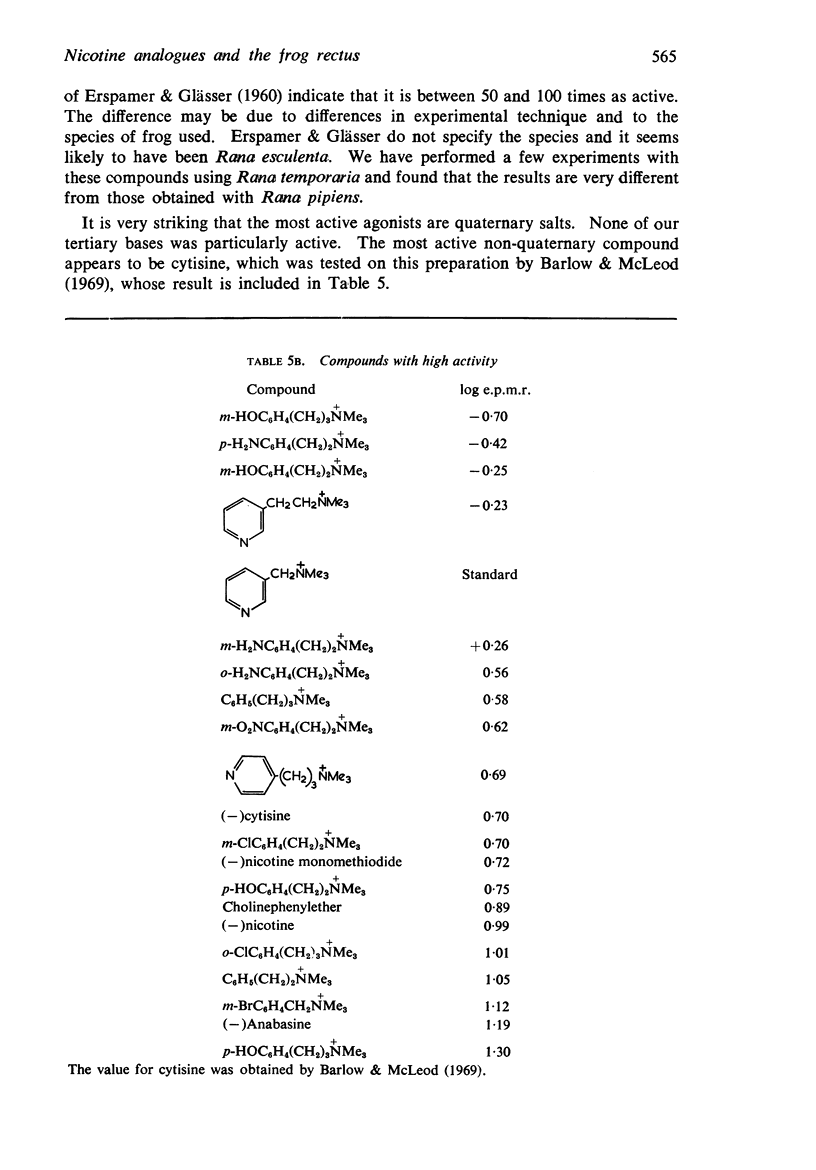
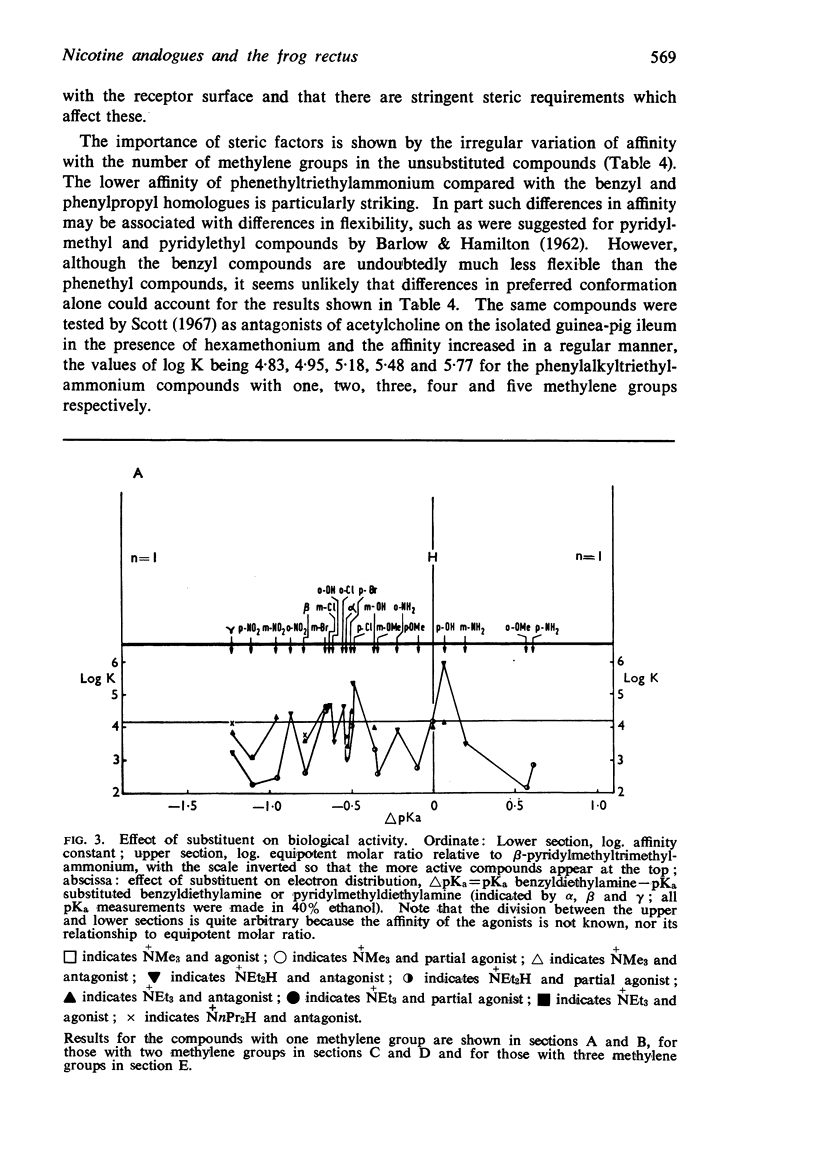
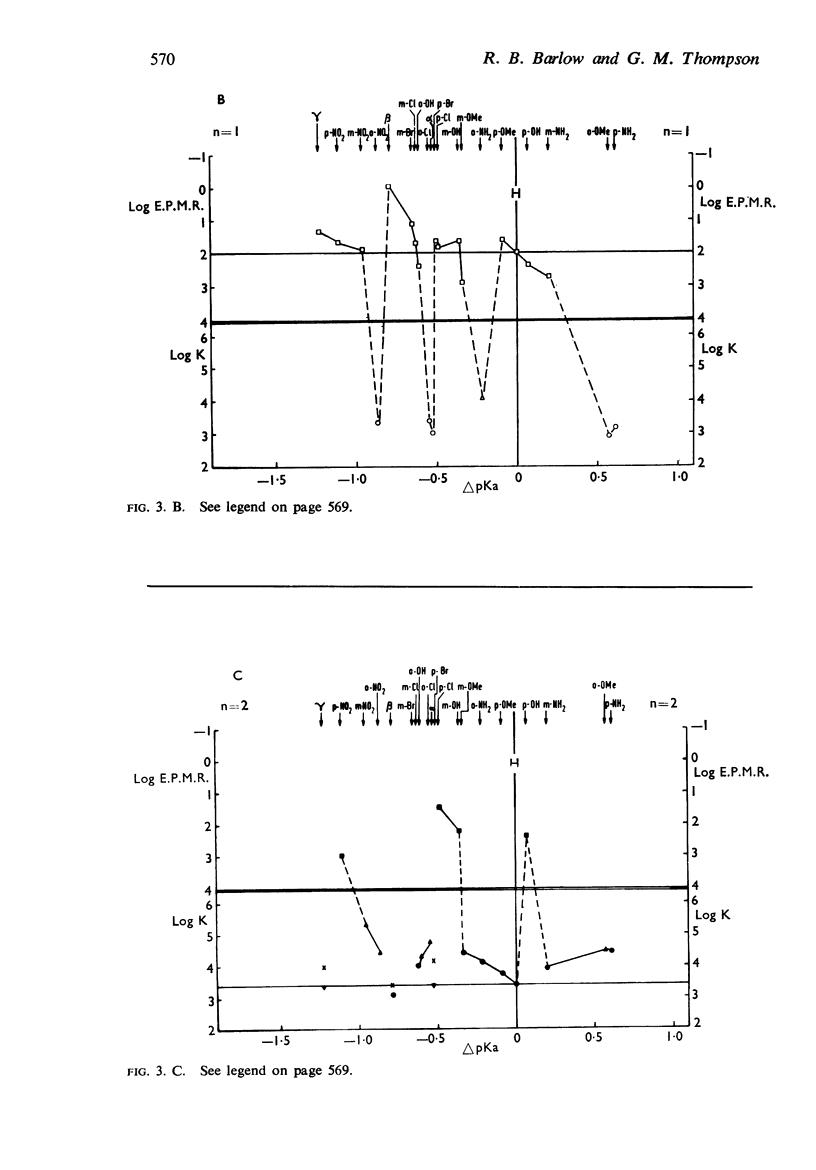
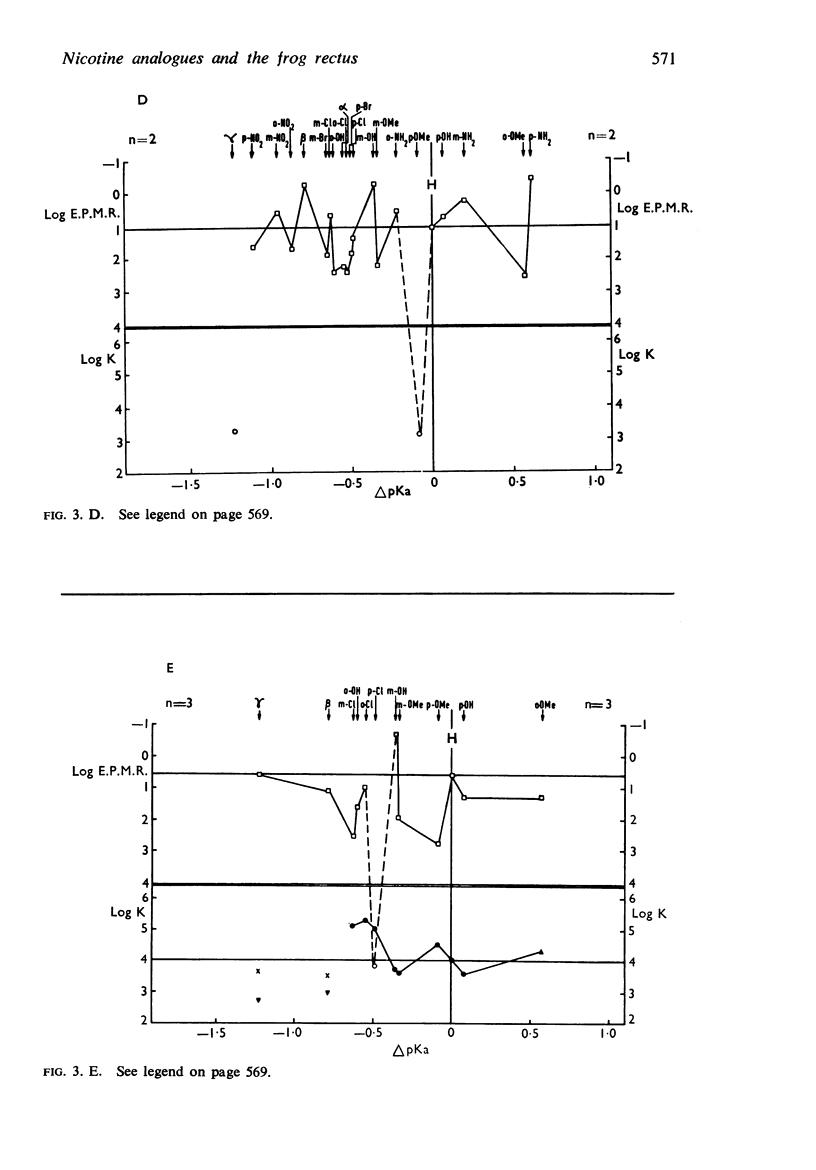
5. There is no obvious correlation between the effects of a substituent on the affinity of the diethylamino or triethylammonium compounds and its effects on the activity of the trimethylammonium analogue. The most active compounds contain hydroxy- and amino-, phenyl or β-pyridyl groups, m-hydroxyphenyl-propyltrimethylammonium being about 50 times as active as nicotine, but the corresponding diethylamino or triethylammonium compounds do not have high affinity. There does not seem necessarily to be an inverse relationship between activity and affinity, however, because some m-nitro and m-chloro trimethylammonium compounds have considerable activity and the analogous triethylammonium compounds have considerable affinity.
6. It is suggested that ability to activate these receptors is associated with the presence of substituents which can interact with water molecules which may be involved in the action of the drug at the receptor.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson F. B., Barlow R. B., Mustafa M. G., Stephenson R. P. Relationships between chemical structure and affinity for acetylcholine receptors. Br J Pharmacol. 1969 Sep;37(1):207–233. doi: 10.1111/j.1476-5381.1969.tb09539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW R. B., DOBSON N. A. Nicotine monomethiodide. J Pharm Pharmacol. 1955 Jan;7(1):27–34. doi: 10.1111/j.2042-7158.1955.tb12000.x. [DOI] [PubMed] [Google Scholar]
- BARLOW R. B., ZOLLER A. SOME EFFECTS OF LONG CHAIN POLYMETHYLENE BISONIUM SALTS ON JUNCTIONAL TRANSMISSION IN THE PERIPHERAL NERVOUS SYSTEM. Br J Pharmacol Chemother. 1964 Aug;23:131–150. doi: 10.1111/j.1476-5381.1964.tb01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., McLeod L. J. Some studies on cytisine and its methylated derivatives. Br J Pharmacol. 1969 Jan;35(1):161–174. doi: 10.1111/j.1476-5381.1969.tb07977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Scott N. C., Stephenson R. P. The affinity and efficacy of onium salts on the frog rectus abdominis. Br J Pharmacol Chemother. 1967 Sep;31(1):188–196. doi: 10.1111/j.1476-5381.1967.tb01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN M. E., HUME A. H., HOLLAND W. C. NICOTINE-LIKE STIMULANT ACTIONS OF SEVERAL SUBSTITUTED PHENYLCHOLINE ETHERS. J Pharmacol Exp Ther. 1965 Apr;148:66–70. [PubMed] [Google Scholar]
- ERSPAMER V., GLASSER A. The pharmacological actions of (m-hydroxyphenethyl) trimethylammonium (leptodactyline). Br J Pharmacol Chemother. 1960 Mar;15:14–22. doi: 10.1111/j.1476-5381.1960.tb01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERSPAMER V. Isolation of leptodactyline (m-hydroxyphenylethyltrimethylammonium) from extracts of Leptodactylus skin. Arch Biochem Biophys. 1959 Jun;82(2):431–438. doi: 10.1016/0003-9861(59)90139-0. [DOI] [PubMed] [Google Scholar]
- HEY P. On relationships between structure and nicotine-like stimulant activity in choline esters and ethers. Br J Pharmacol Chemother. 1952 Mar;7(1):117–129. doi: 10.1111/j.1476-5381.1952.tb00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglid F. Studies on pyridine alkaloids and their analogues. Acta Pharm Suec. 1967 Apr;4(2):117–138. [PubMed] [Google Scholar]
- Kier L. B. A molecular orbital calculation of the preferred conformation of nicotine. Mol Pharmacol. 1968 Jan;4(1):70–76. [PubMed] [Google Scholar]
- ORMEROD W. E. The pharmacology of benzoylcholine derivatives and the nature of carbonyl receptors. Br J Pharmacol Chemother. 1956 Sep;11(3):267–272. doi: 10.1111/j.1476-5381.1956.tb01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKUL A. A., HOLLAND W. C. Comparative pressor effect of certain unsaturated acid esters of choline. J Pharmacol Exp Ther. 1961 Sep;133:313–318. [PubMed] [Google Scholar]
- SEKUL A. A., HOLLAND W. C. Pharmacology of senecioyl-choline. J Pharmacol Exp Ther. 1961 May;132:171–175. [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS J., MARLOW W. QUATERNARY AMMONIUM COMPOUNDS. 3. ANTIACETYLCHOLINESTERASE ACTIVITY AND CHARGE DISTRIBUTION IN AROMATIC QUATERNARY AMMONIUM COMPOUNDS. J Med Chem. 1963 Mar;6:107–111. doi: 10.1021/jm00338a005. [DOI] [PubMed] [Google Scholar]
- WONG K. C., LONG J. P. Nicotinic and muscarinic activity of phenacyl and phenylalkyl trimethylamines. J Pharmacol Exp Ther. 1962 Jul;137:70–75. [PubMed] [Google Scholar]


