Abstract
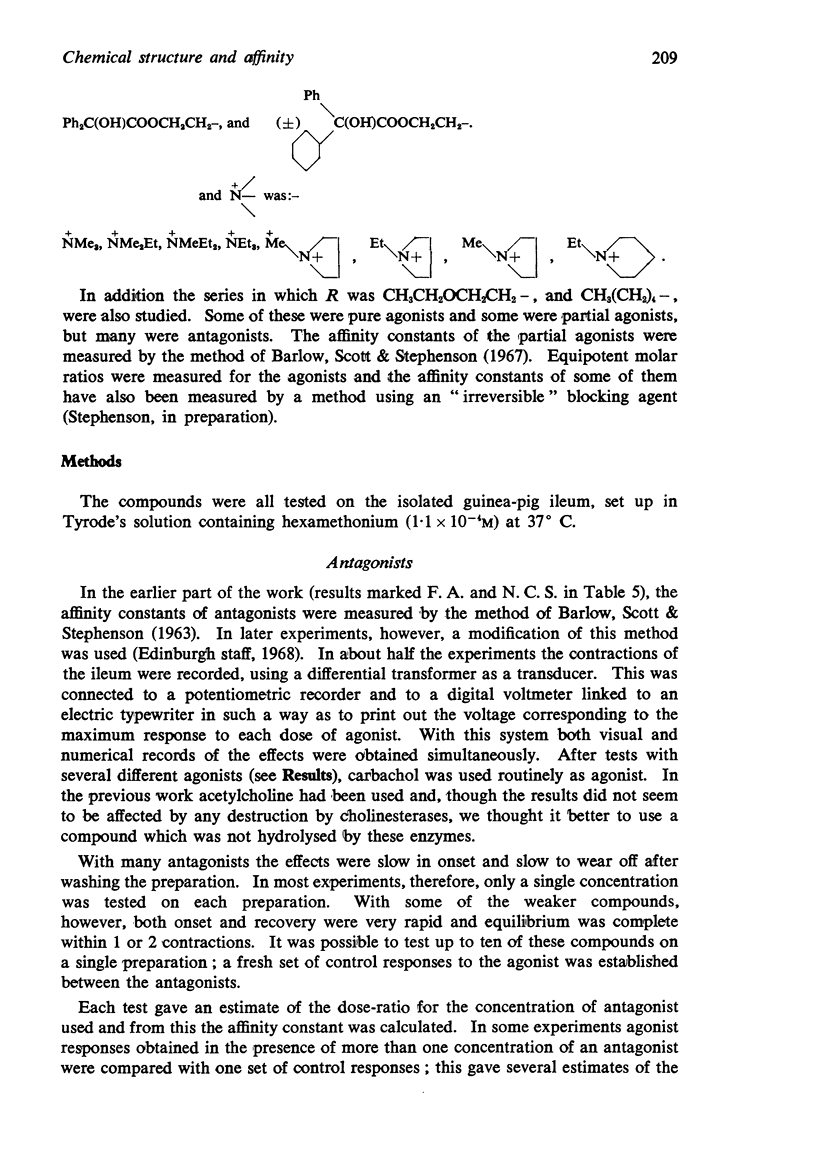
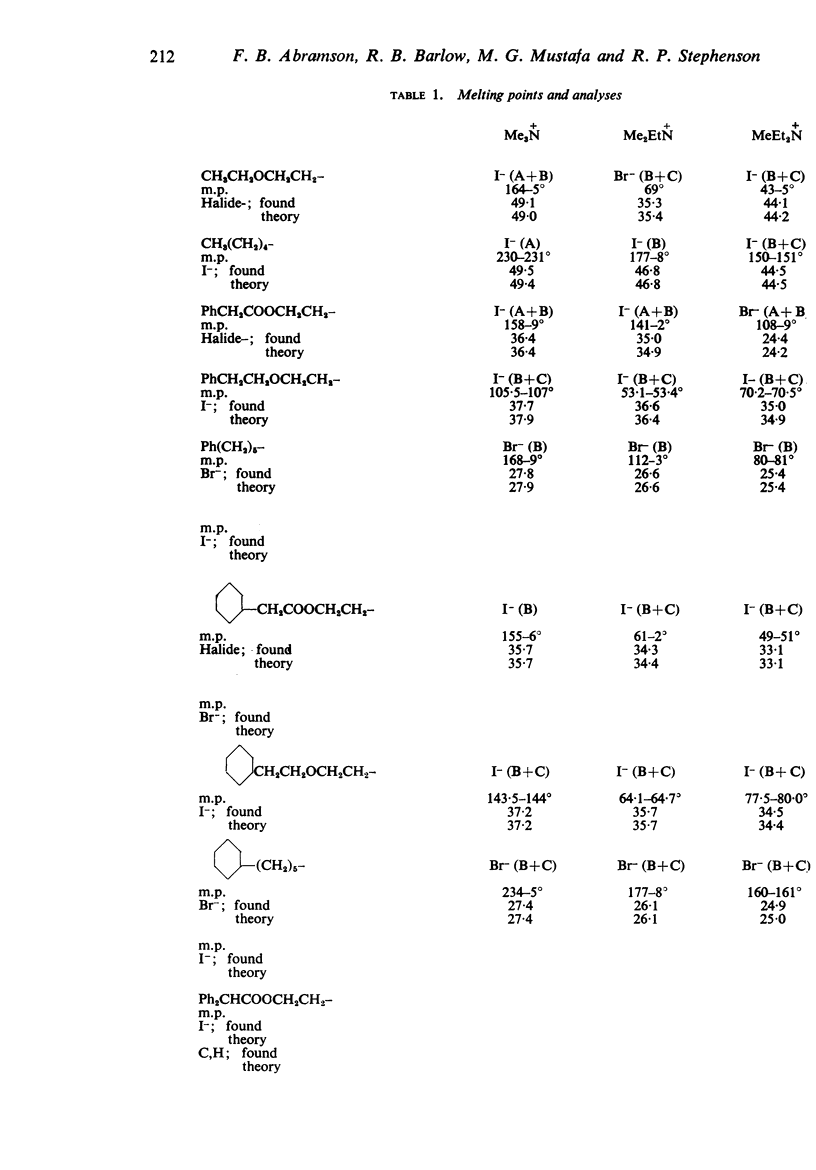
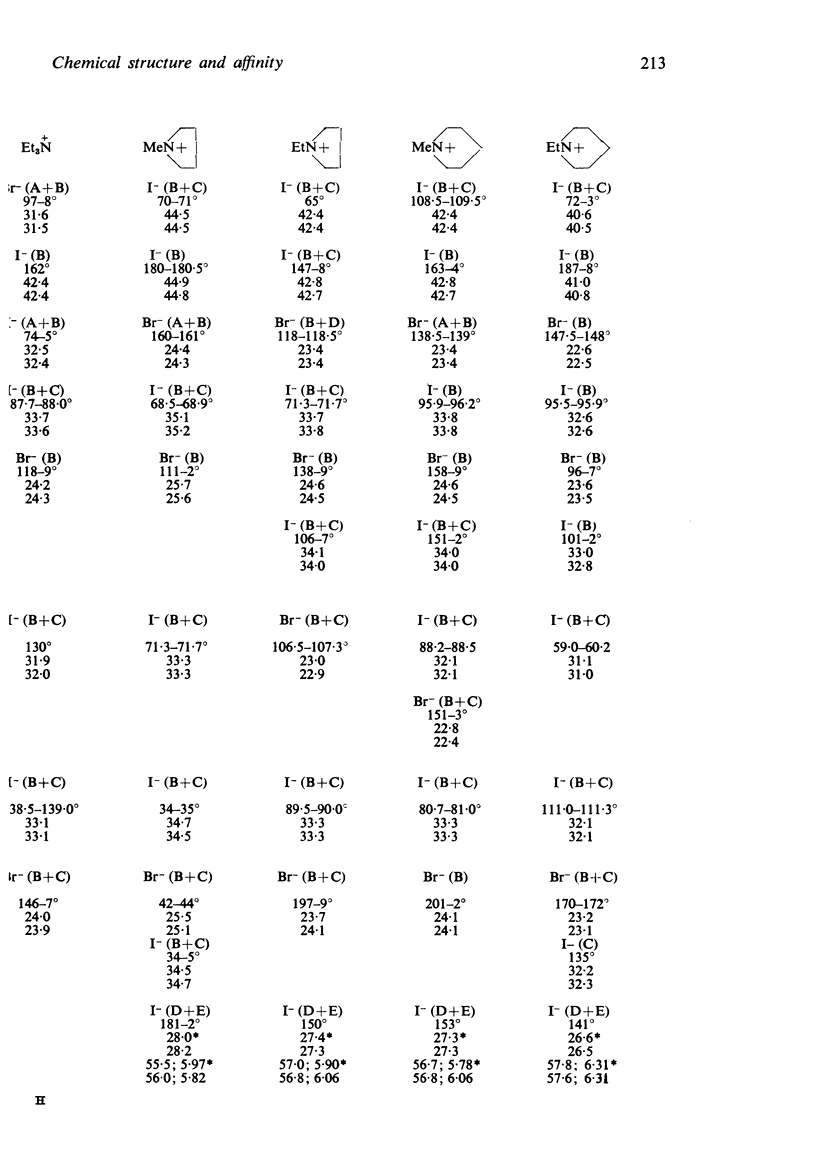
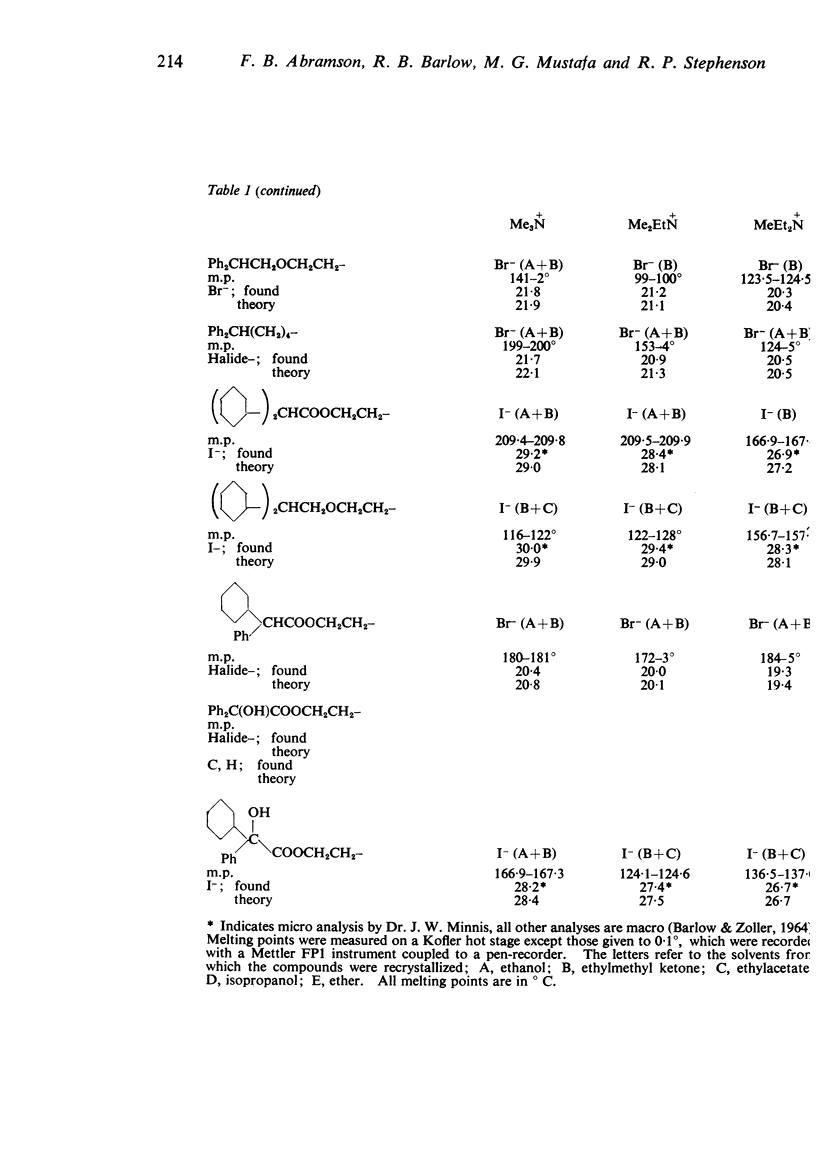
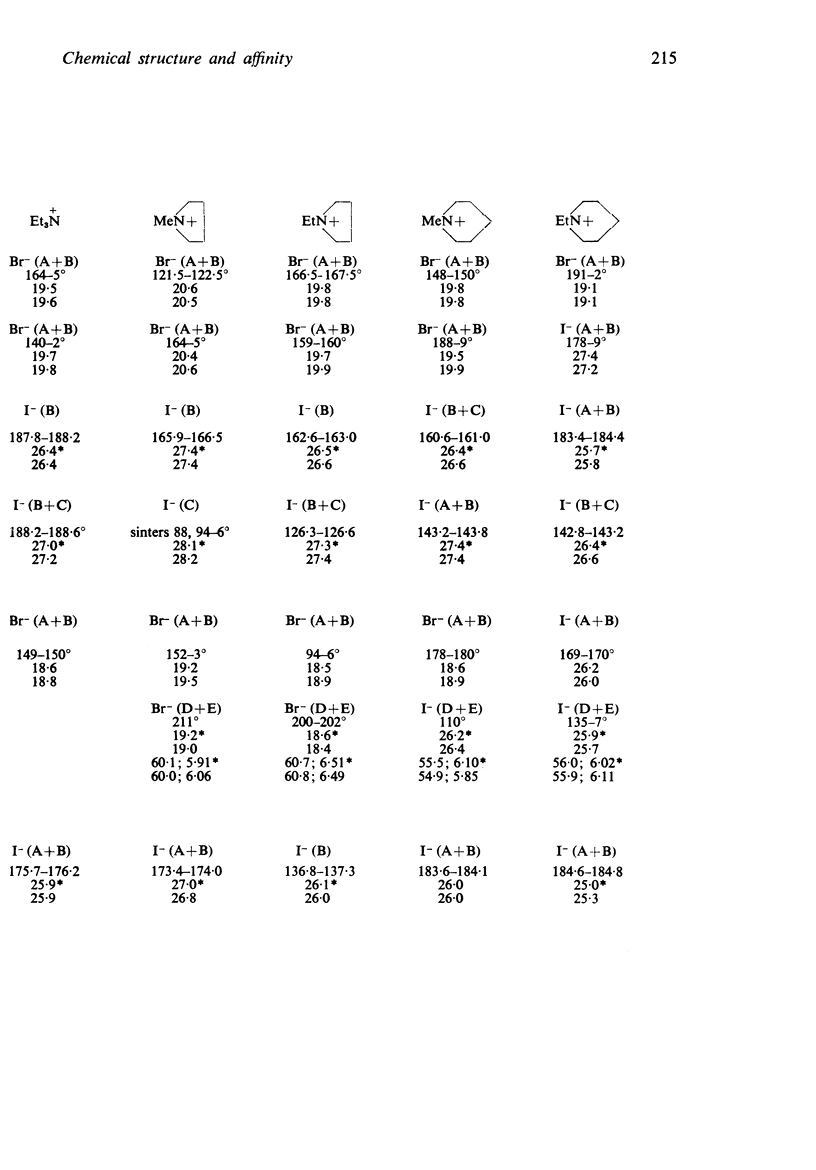
1. Series of analogues of acetylcholine have been prepared in which the acetyl group was replaced by phenylacetyl, cyclohexylacetyl, diphenylacetyl, dicyclohexylacetyl, (±)-phenylcyclohexylacetyl, benziloyl and (±)-phenylcyclohexylhydroxyacetyl groups and the trimethylammonium group was replaced by Me2EtN+, MeEt2N+, Et3N+, [Formula: see text] Further series were prepared in which the acetoxyethyl group was replaced by ethoxyethyl, phenylethoxyethyl, cyclohexylethoxyethyl, diphenylethoxyethyl, and dicyclohexylethoxyethyl groups, and by n-pentyl, 5-phenylpentyl, 5-cyclohexylpentyl and 5:5-diphenylpentyl groups.
2. The ethoxyethyl and n-pentyl series contain some compounds which are agonists or partial agonists when tested on the isolated guinea-pig ileum, but all the other compounds are antagonists.
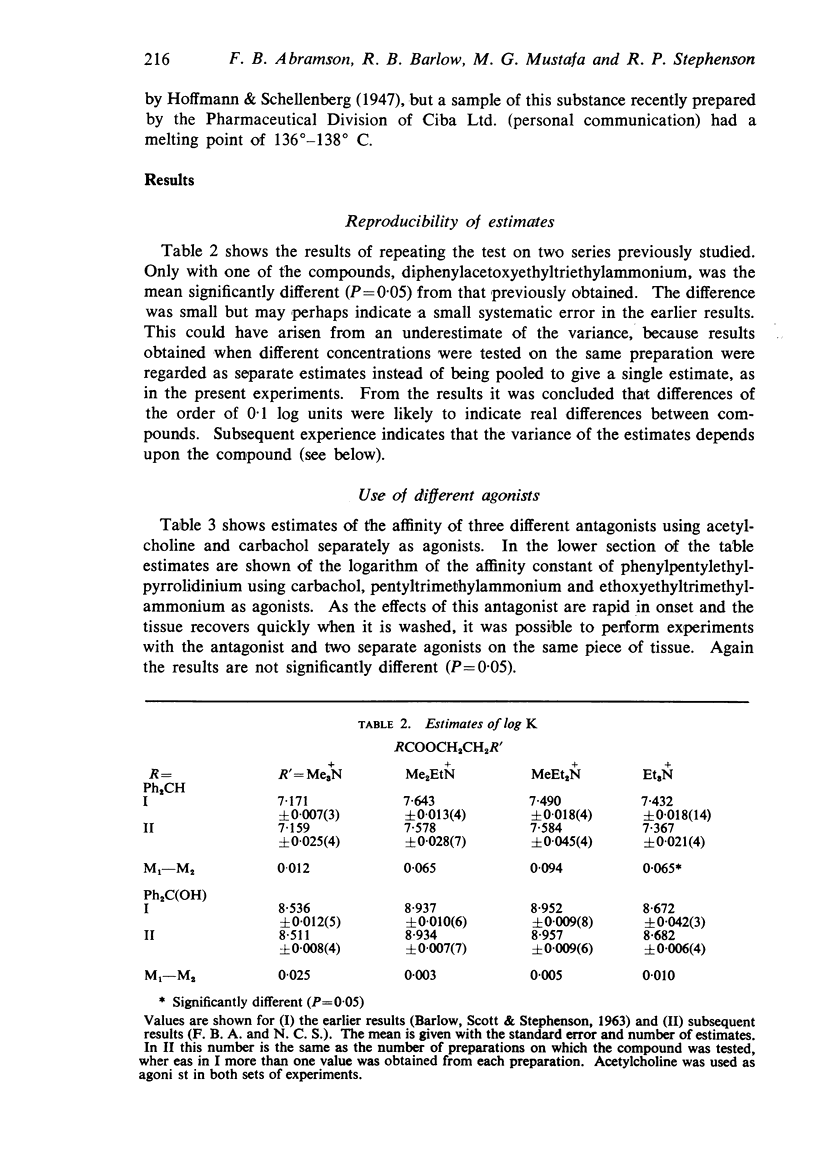
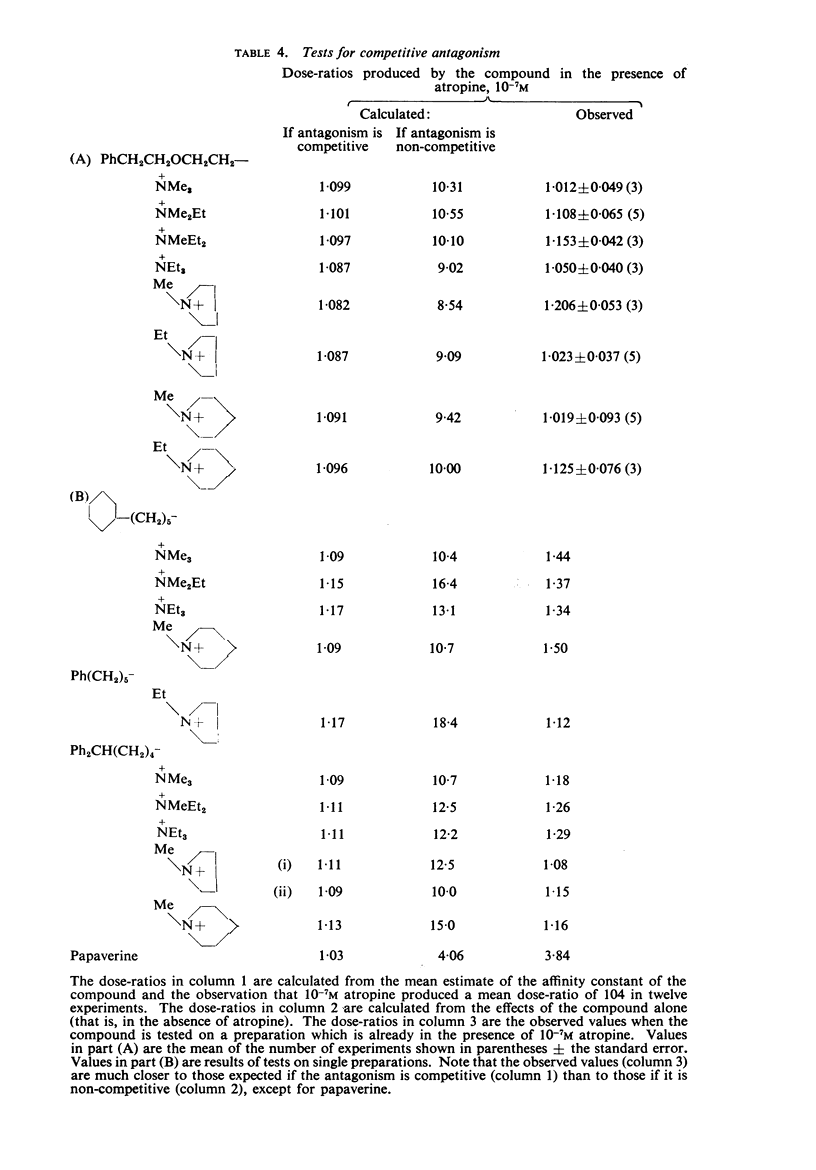
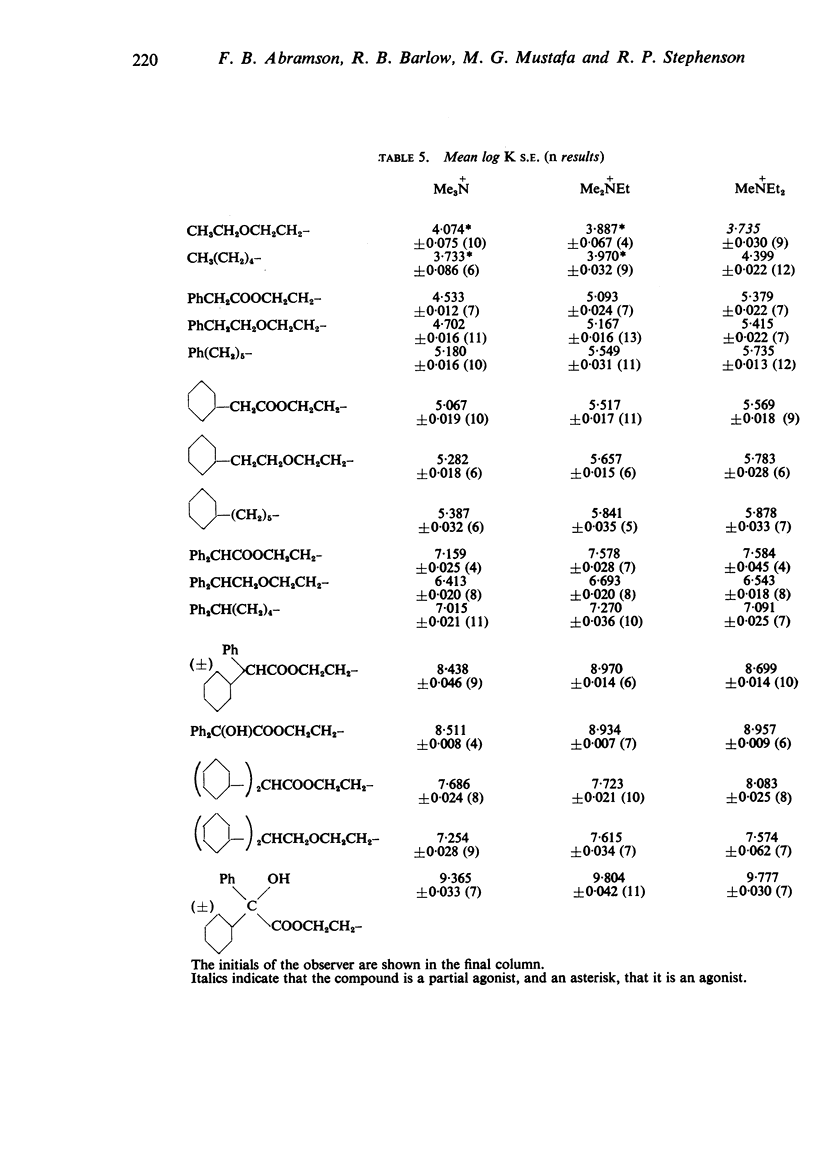
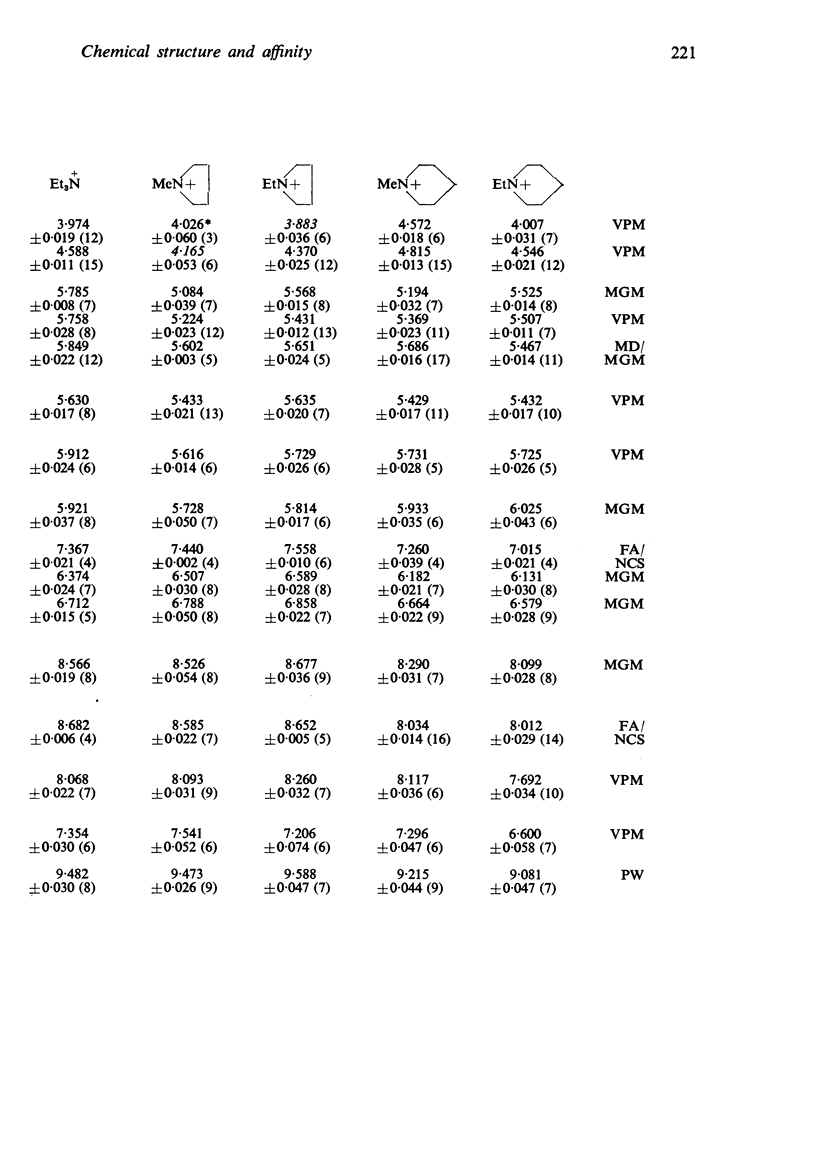
3. The affinity of the compounds for the postganglionic (“muscarinesensitive”) acetylcholine receptors has been measured in conditions in which the antagonists have been shown to be acting competitively. There were considerable differences between their affinities, the most active (log K, 9·8) having one million times the affinity of the least active (log K, 3·7).
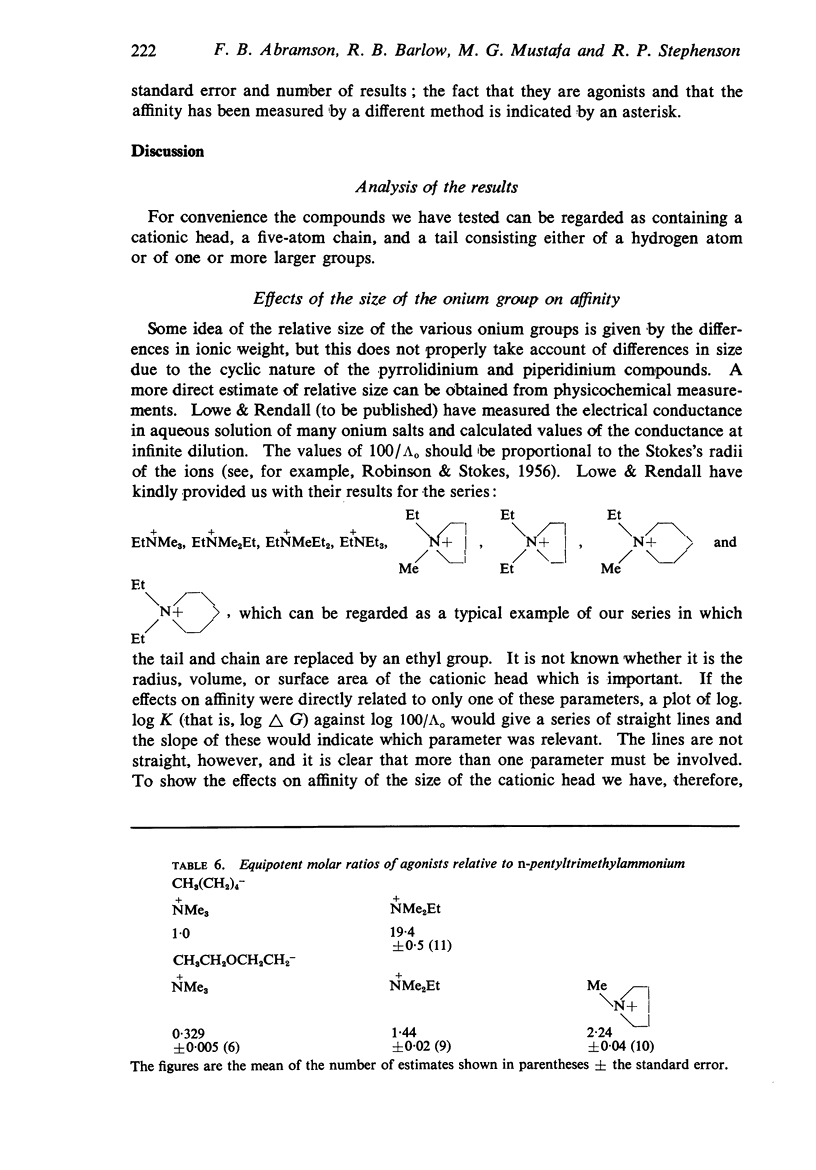
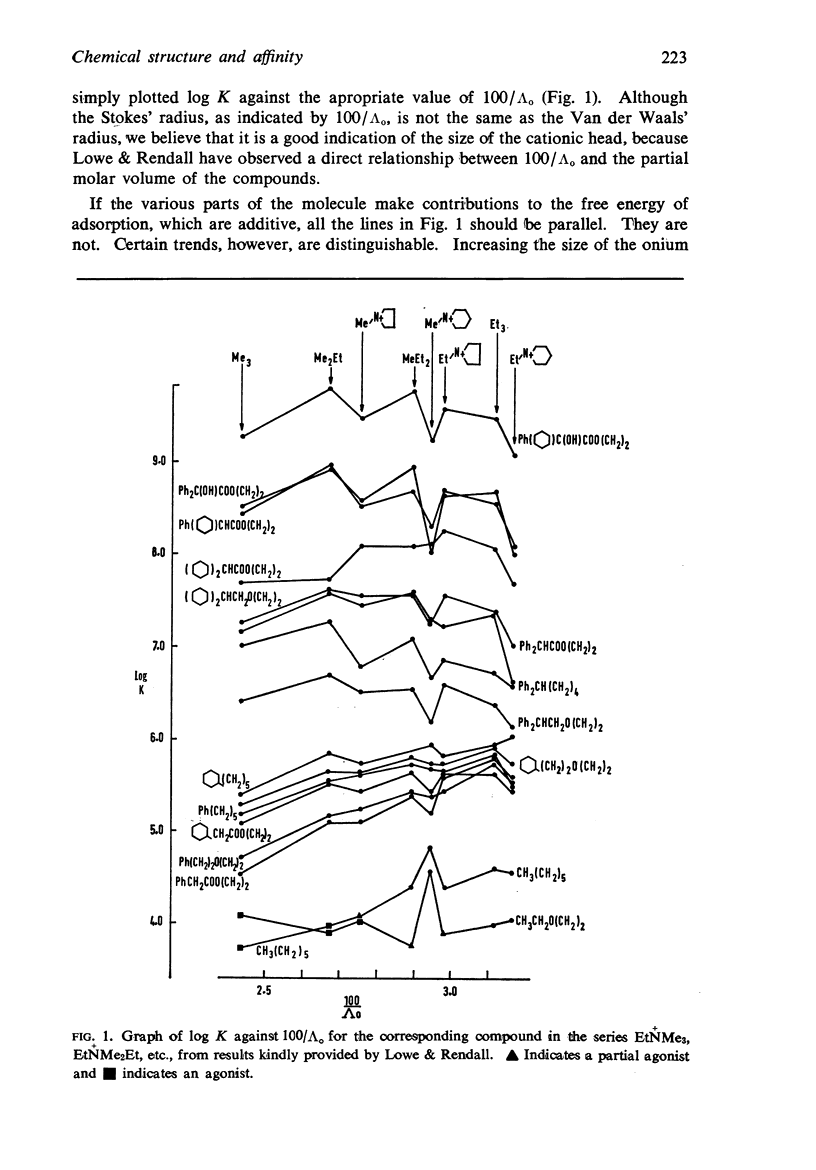
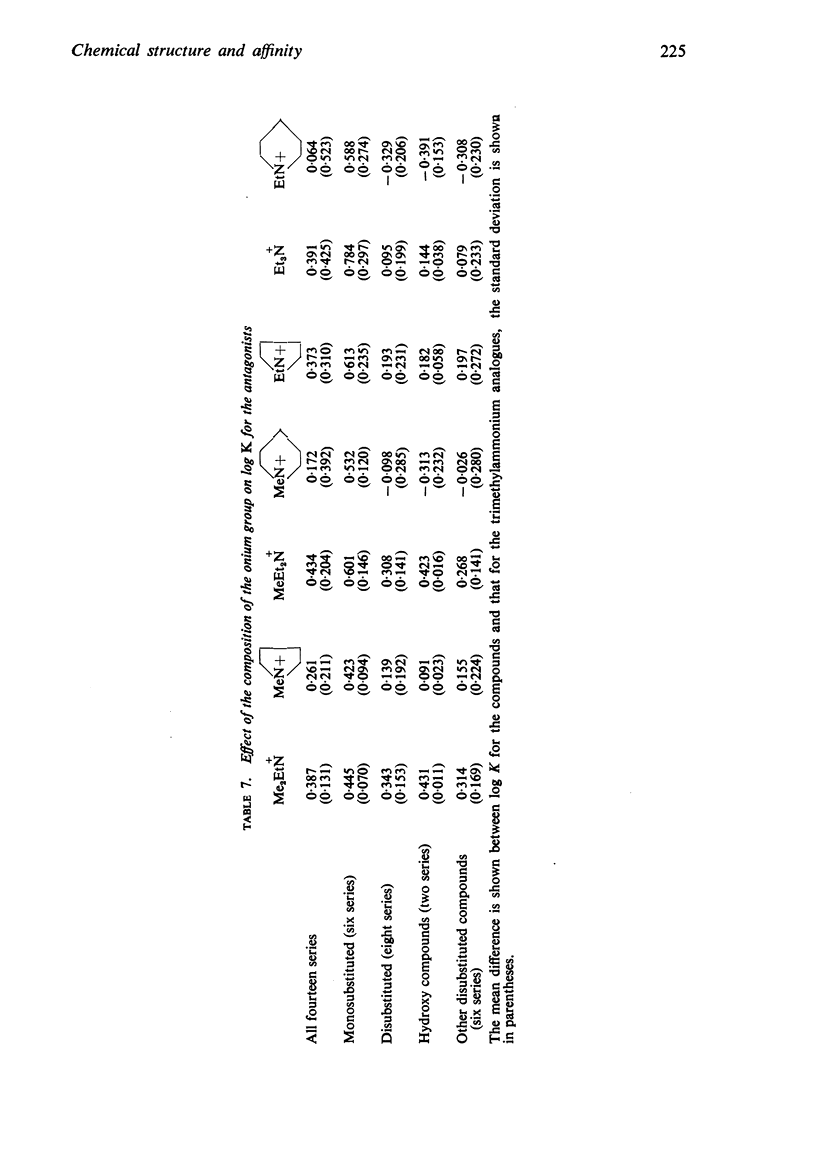
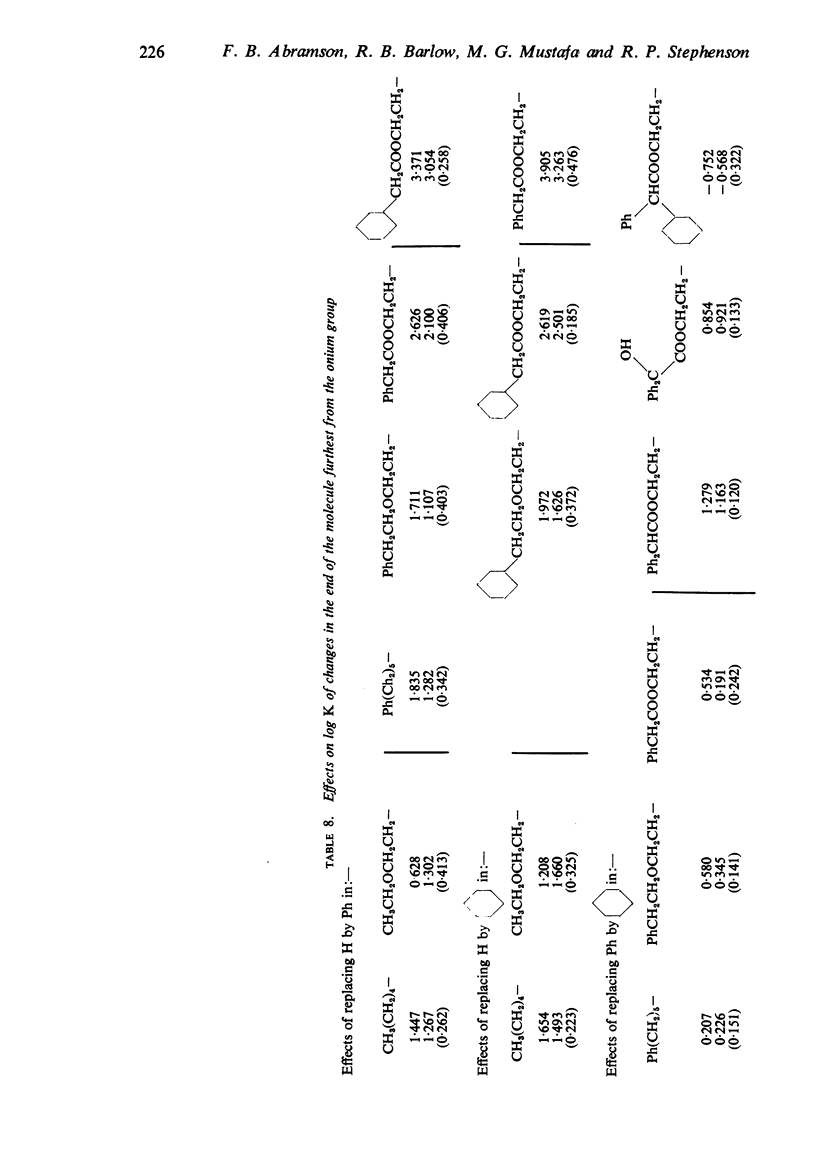
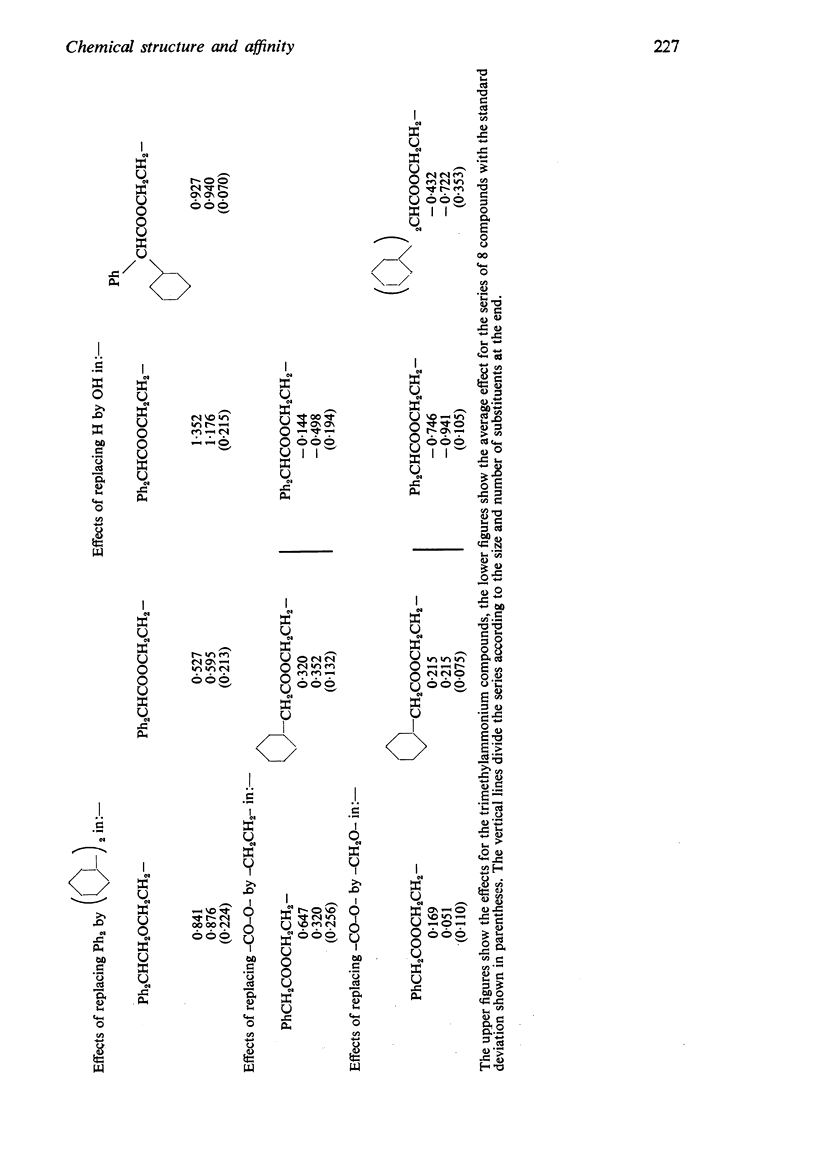
4. The changes in affinity as the onium group was modified were not entirely independent of changes in the rest of the molecule. Increasing the size of the onium group, as judged from conductivity measurements on simpler onium salts, increased affinity in the series containing one large group (phenyl or cyclohexyl) but, in the series with two large groups, affinity declined when the size was increased beyond -+NMeEt2.
5. In general, the effects of changes in the rest of the molecule on affinity were bigger than the effects of changes in the onium group and there were bigger interactions. Affinity was increased to a greater extent by introducing one phenyl and one cyclohexyl group together than by introducing either two phenyl or two cyclohexyl groups; the increment was greater than the separate contributions made by one phenyl and one cyclohexyl group.
6. The factors which influence the binding of molecules to receptors are discussed. There is no evidence that the separation between the onium group and the group in the receptor with which it interacts is greater in compounds with high affinity nor is there any evidence, from the study of the series which contain agonists and partial agonists, that ability to activate receptors depends upon the onium group being able to come close to this charged group in the receptors.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW R. B., SCOTT K. A., STEPHENSON R. P. AN ATTEMPT TO STUDY THE EFFECTS OF CHEMICAL STRUCTURE ON THE AFFINITY AND EFFICACY OF COMPOUNDS RELATED TO ACETYLCHOLINE. Br J Pharmacol Chemother. 1963 Dec;21:509–522. doi: 10.1111/j.1476-5381.1963.tb02019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW R. B., ZOLLER A. SOME EFFECTS OF LONG CHAIN POLYMETHYLENE BISONIUM SALTS ON JUNCTIONAL TRANSMISSION IN THE PERIPHERAL NERVOUS SYSTEM. Br J Pharmacol Chemother. 1964 Aug;23:131–150. doi: 10.1111/j.1476-5381.1964.tb01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Scott N. C., Stephenson R. P. The affinity and efficacy of onium salts on the frog rectus abdominis. Br J Pharmacol Chemother. 1967 Sep;31(1):188–196. doi: 10.1111/j.1476-5381.1967.tb01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek B. W., Nivard R. J., van Rossum J. M., Ariëns E. J. Absolute configuration and parasympathetic action: pharmacodynamics of enantiomorphic and diastereoisomeric esters of beta-methylcholine. J Pharm Pharmacol. 1965 Jul;17(7):393–404. doi: 10.1111/j.2042-7158.1965.tb07694.x. [DOI] [PubMed] [Google Scholar]
- GADDUM J. H. Theories of drug antagonism. Pharmacol Rev. 1957 Jun;9(2):211–218. [PubMed] [Google Scholar]
- PATON W. D., RANG H. P. THE UPTAKE OF ATROPINE AND RELATED DRUGS BY INTESTINAL SMOOTH MUSCLE OF THE GUINEA-PIG IN RELATION TO ACETYLCHOLINE RECEPTORS. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]


