Abstract
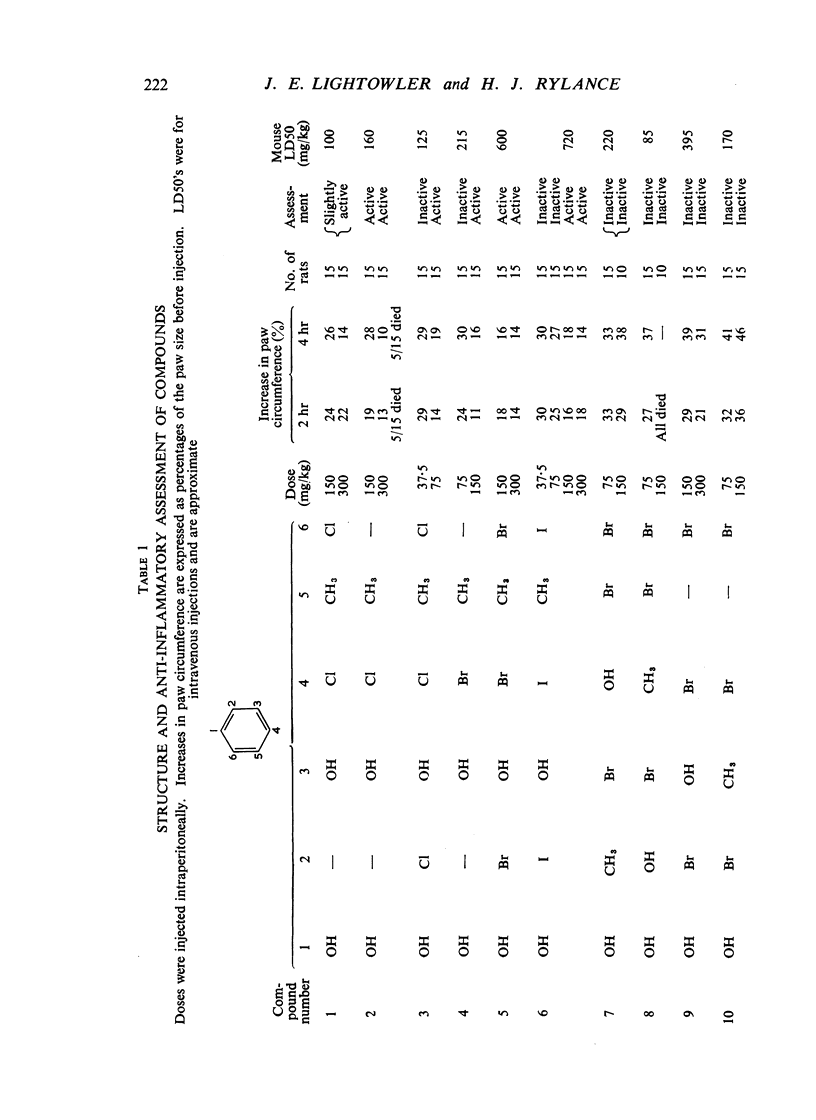
Some substituted dihydroxybenzenes have been examined for suppression of the yeast-induced inflammatory reaction in the rat paw. This anti-inflammatory activity is greatest in those compounds derived from resorcinol and in particular the halogenated 5-methylresorcinols. The significance of the internuclear distance between the hydroxyl groups in the meta-position to each other in resorcinol and the importance of the enhanced activity due to the halogen atom(s) is discussed. In this series the toxicity and activity could not be divorced.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LIGHTOWLER J. E., RYLANCE H. J. SUBSTITUTED DIHYDROXYBENZOIC ACIDS AS POSSIBLE ANTI-INFLAMMATORY AGENTS. J Pharm Pharmacol. 1963 Oct;15:633–638. doi: 10.1111/j.2042-7158.1963.tb12853.x. [DOI] [PubMed] [Google Scholar]
- REID J., WATSON R. D., COCHRAN J. B., SPROULL D. H., CLAYTON B. E., PRUNTY F. T. G. Sodium gamma-resorcylate in rheumatic fever. Br Med J. 1951 Aug 11;2(4727):321–326. doi: 10.1136/bmj.2.4727.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDER C. V., WAX J., SCOTTI L., SCHERRER R. A., JONES E. M., SHORT F. W. Anti-inflammatory, antipyretic and antinociceptive properties of N-(2,3-xylyl)anthranilic acid (mefenamic acid). J Pharmacol Exp Ther. 1962 Dec;138:405–413. [PubMed] [Google Scholar]


