Abstract
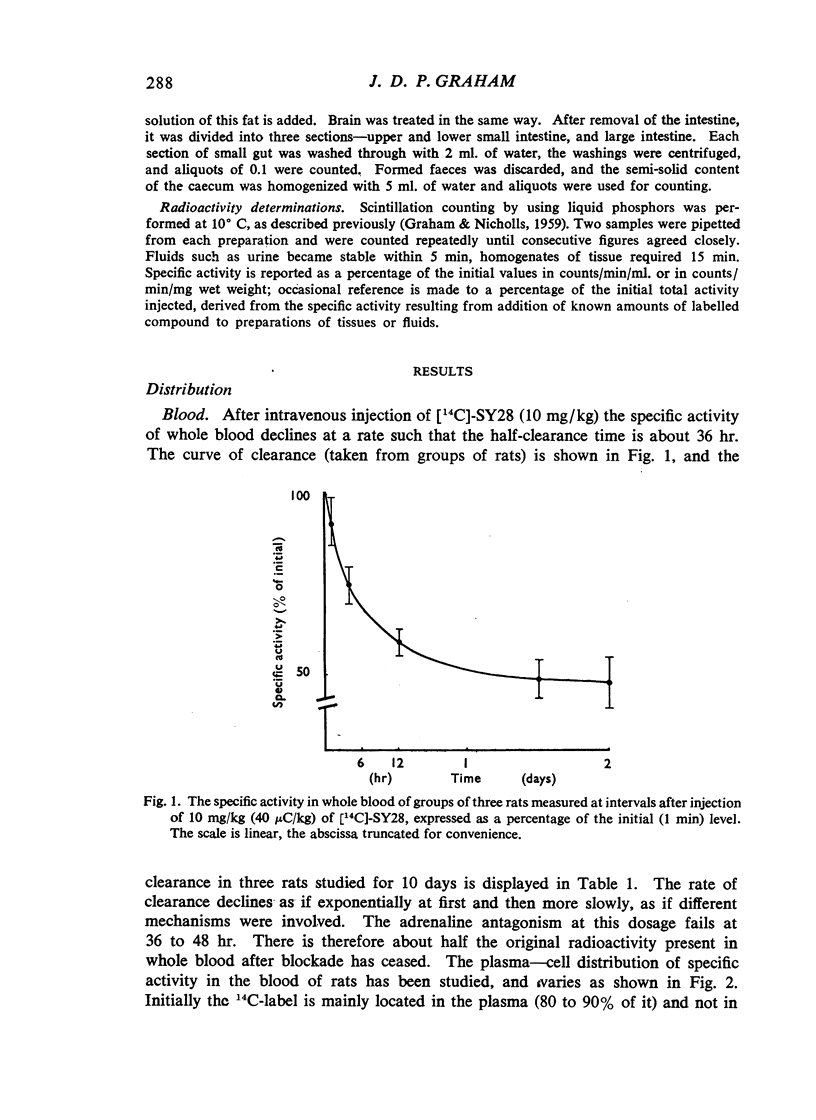
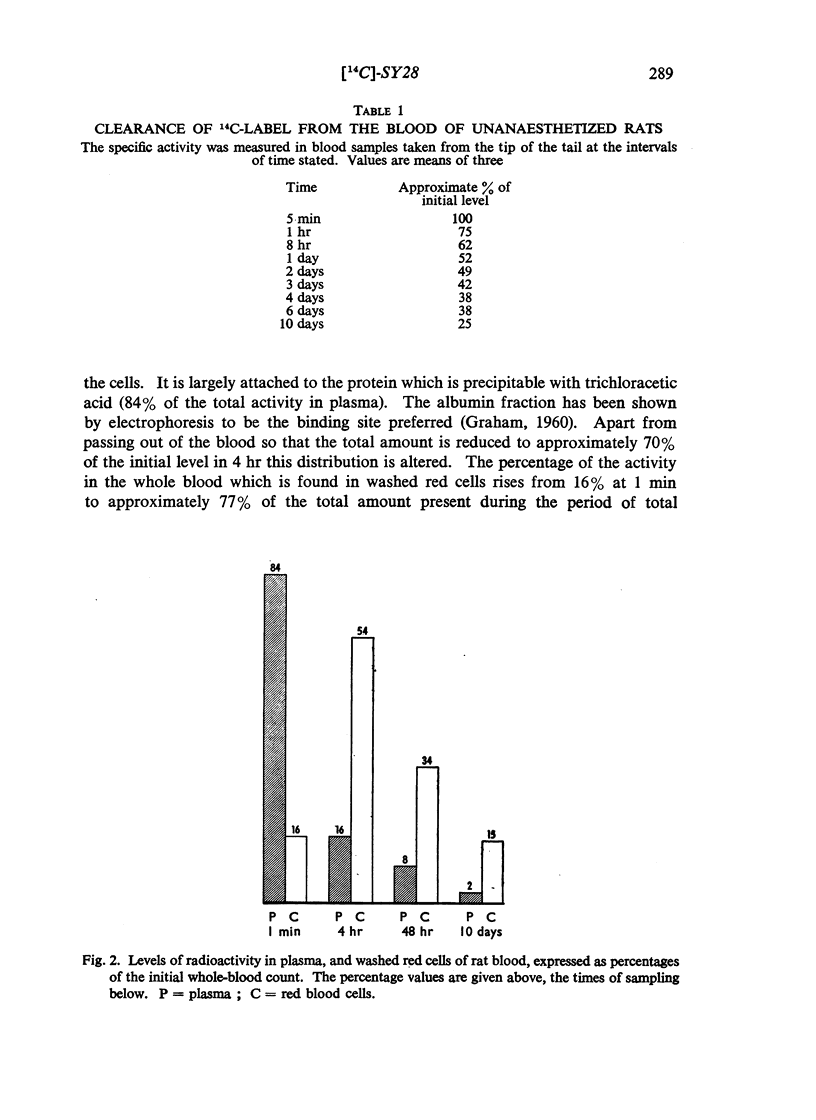
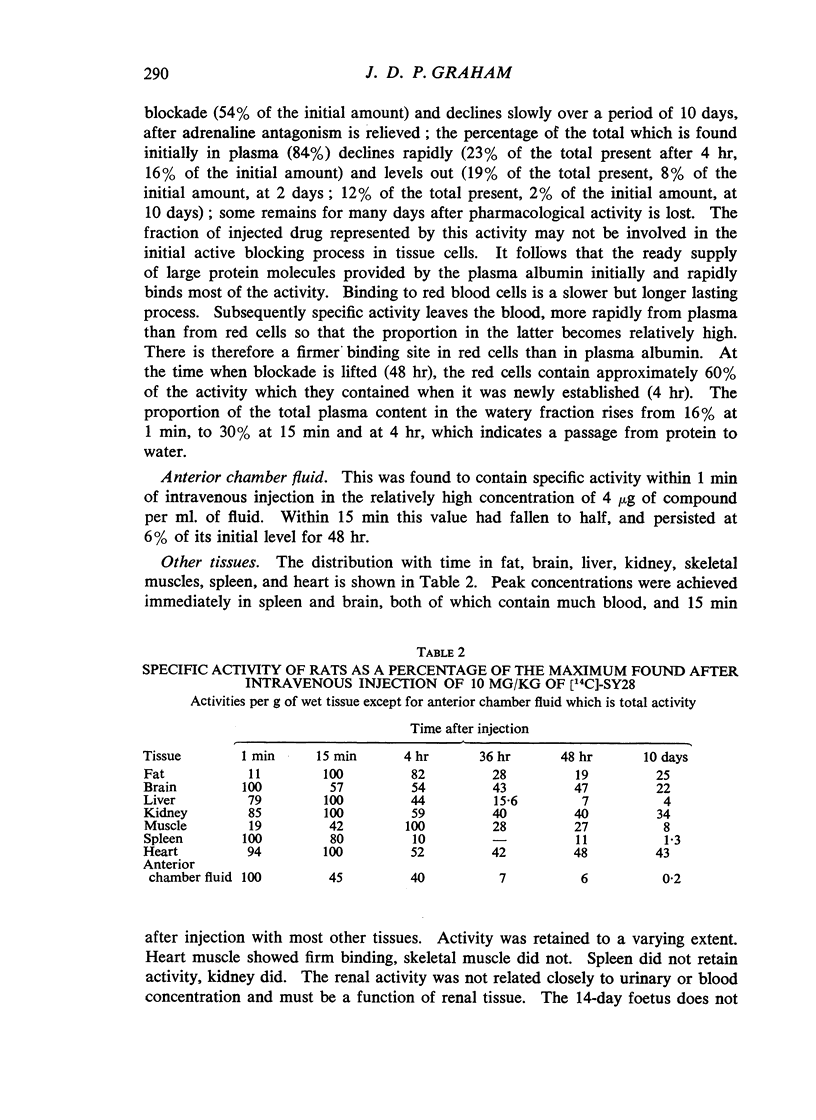
(2-Bromoethyl)ethyl(naphth-1-ylmethyl)amine hydrobromide (SY28) is a halogenoalkylamine related to dibenamine. A dose of 10 mg/kg injected intravenously into rats antagonizes the pressor response to adrenaline for 36 hr. If this amount of SY28 labelled with 14C in the 1-methyl position is administered, specific radioactivity is present in blood and tissues many days after the antagonism of adrenaline is relieved. The 14C is excreted in bile and in urine, but not in expired air. It is present in fat but not to a greater extent than it is in other tissues. It does not cross the placental barrier. There is no evidence that slow release from a lipid depot accounts for the long duration of action.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., ARONOW L., BRODIE B. B. The physiological disposition and biotransformation of dibenamine and a method for its estimation in biological tissues. J Pharmacol Exp Ther. 1952 Oct;106(2):166–179. [PubMed] [Google Scholar]
- BRODIE B. B., ARONOW L., AXELROD J. The fate of dibenzyline in the body and the role of fat in its duration of action. J Pharmacol Exp Ther. 1954 May;111(1):21–29. [PubMed] [Google Scholar]
- GRAHAM J. D., LEWIS G. P. The antihistamine and antiadrenaline properties of a series of N-naphthylmethyl-2-haloethylamine derivatives. Br J Pharmacol Chemother. 1953 Mar;8(1):54–61. doi: 10.1111/j.1476-5381.1953.tb00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM J. D., LEWIS G. P. The role of the cyclic ethyleneiminium ion in the pharmacological activity of the 2-haloethylamines. Br J Pharmacol Chemother. 1954 Mar;9(1):68–75. doi: 10.1111/j.1476-5381.1954.tb00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM J. D., NICHOLLS P. J. Distribution of [14C] bemegride in tissues after intravenous injection. Br J Pharmacol Chemother. 1959 Mar;14(1):35–39. doi: 10.1111/j.1476-5381.1959.tb00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM J. D. The ethyleneiminium ion as the active species in 2-haloalkylamine compounds. Br J Pharmacol Chemother. 1957 Dec;12(4):489–497. doi: 10.1111/j.1476-5381.1957.tb00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM J. D. [Pharmacology and therapeutic uses of the adrenomotor antagonists of the 2-halogenoalkylamines series]. Recenti Prog Med. 1960 Jun;28:487–507. [PubMed] [Google Scholar]
- KUSCHINSKY G., VORHERR H. Die Adrenalin-Umkehr durch Adrenolytica am Blutdruck der Maus. Arzneimittelforschung. 1958 Aug;8(8):484–486. [PubMed] [Google Scholar]
- NICKERSON M. Mechanism of the prolonged adrenergic blockade produced by haloalkylamines. Arch Int Pharmacodyn Ther. 1962 Nov 1;140:237–250. [PubMed] [Google Scholar]


