Abstract
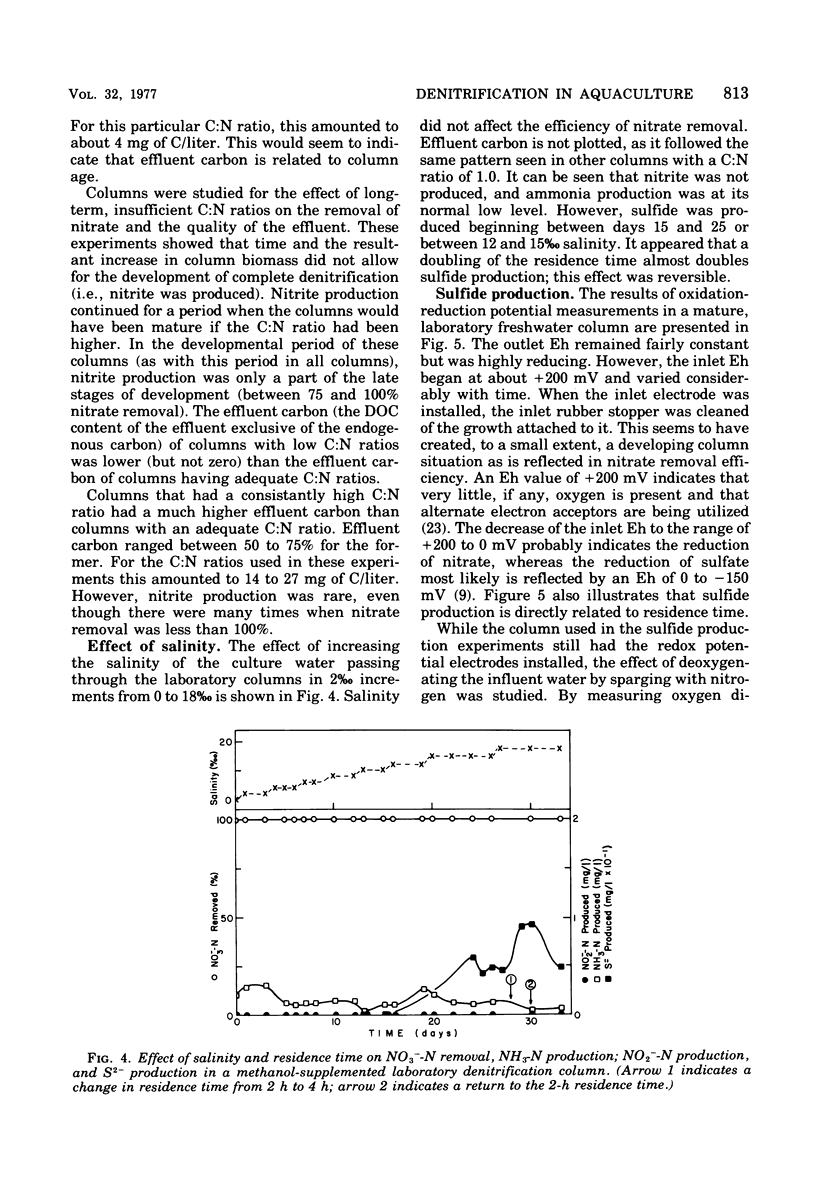
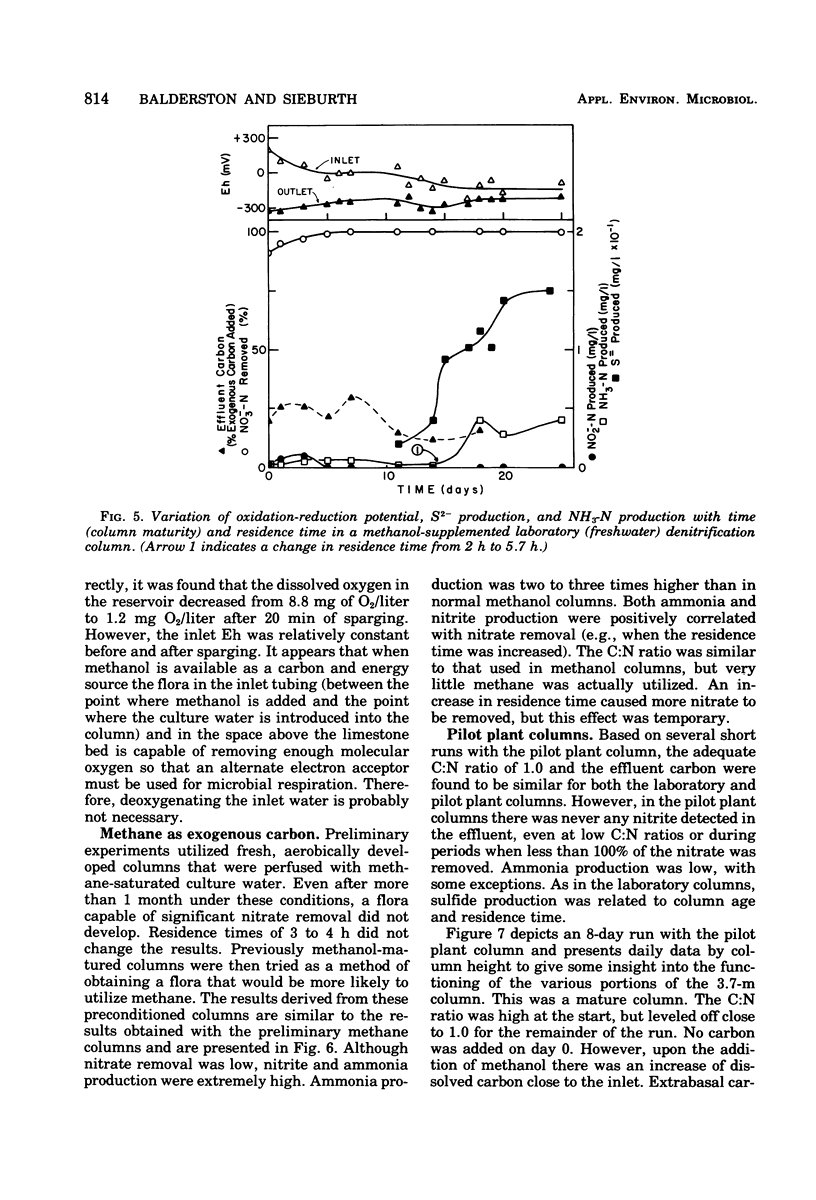
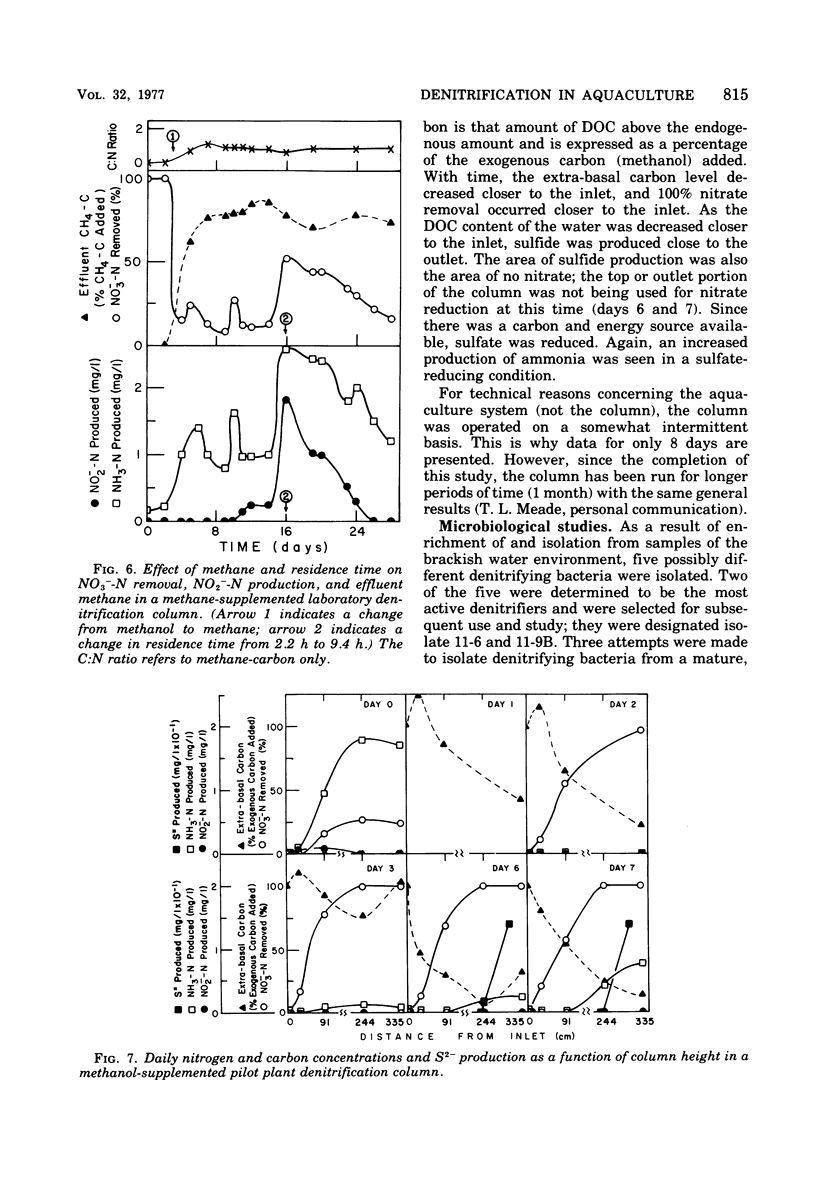
The columnar denitrification method of nitrate-nitrogen removal from high-density, closed system, salmonid aquaculture was investigated and found to be feasible. However, adequate chemical monitoring was found to be necessary for the optimization and quality control of this method. When methanol-carbon was not balanced with inlet nitrate-nitrogen, the column effluent became unsatisfactory for closed-system fish culture due to the presence of excess amounts of nitrite, ammonia, sulfide, and dissolved organic carbon. Sulfide production was also influenced by column maturity and residence time. Methane-carbon was found to be unsatisfactory as an exogenous carbon source. Endogenous carbon could not support high removal efficiencies. Freshwater columns adpated readily to an artificial seawater with a salinity of 18% without observable inhibition. Scanning electron microscopy revealed that the bacterial flora was mainly rod forms with the Peritricha (protozoa) dominating as the primary consumers. Denitrifying bacteria isolated from freshwater columns were tentatively identified as species of Pseudomonas and Alcaligenes. A pilot plant column was found to behave in a manner similar to the laboratory columns except that nitrite production was never observed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann L., Baumann P., Mandel M., Allen R. D. Taxonomy of aerobic marine eubacteria. J Bacteriol. 1972 Apr;110(1):402–429. doi: 10.1128/jb.110.1.402-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finstein M. S. Nitrogen and phosphorus removal from combined sewage components by microbial activity. Appl Microbiol. 1966 Jul;14(4):679–684. doi: 10.1128/am.14.4.679-684.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Specific inhibitors of ammonia oxidation in Nitrosomonas. J Bacteriol. 1973 Aug;115(2):480–485. doi: 10.1128/jb.115.2.480-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIFSON E. DETERMINATION OF CARBOHYDRATE METABOLISM OF MARINE BACTERIA. J Bacteriol. 1963 May;85:1183–1184. doi: 10.1128/jb.85.5.1183-1184.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell M. R., Doebbler G. F., Hamilton R. W., Jr Serum enzyme level changes in pigs following decompression trauma. Aerosp Med. 1974 May;45(5):519–524. [PubMed] [Google Scholar]
- Ryther J. H., Dunstan W. M. Nitrogen, phosphorus, and eutrophication in the coastal marine environment. Science. 1971 Mar 12;171(3975):1008–1013. doi: 10.1126/science.171.3975.1008. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]