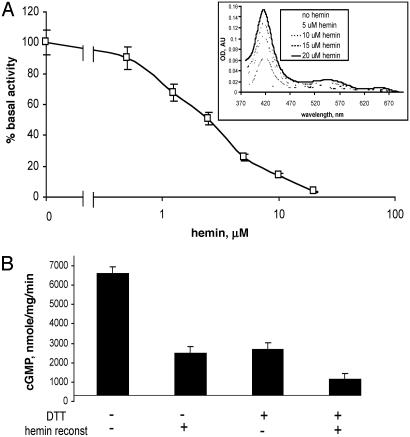
Fig. 3.
Hemin-dependent inhibition of the mutant enzyme. (A) αβCys-105 (4 μM) was supplied with 0.5–20 μM hemin, and specific activity at each hemin concentration was determined and normalized to basal 7.6 ± 0.3 μmol/min per mg activity of the mutant specified as 100%. (Inset) Difference spectra of reconstituted enzyme recorded in 50 mM TEA, pH 7.4/200 mM NaCl. AU, arbitrary units. (B) Specific activity of the purified mutant was determined in the reaction buffers containing 1 mM DTT, 2μM hemin, or both. Data are shown as means ± SD of four independent experiments performed in triplicate.