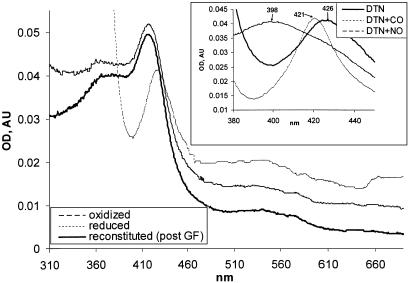
Fig. 4.
Heme reconstitution of αβCys-105 enzyme. (A) αβCys-105 (4 μM) enzyme was supplied with 10 μM hemin and, after 15 min at 24°C, passed through a Hi-Trap desalting column (Amersham Biosciences). The UV-Vis spectra of reconstituted enzyme (solid line) were recorded in 50 mM TEA, pH 7.4, with 200 mM NaCl/10% glycerol. The spectra of reconstituted αβCys-105 oxidized with 5 μM K3[Fe(CN)6] (interrupted line) or reduced by several grains of dithionate (dotted line) are shown. (Inset) Changes in the Soret region of dithionate-reduced hemin-reconstituted αβCys-105 mutant (DTN, solid line) after 15 min of treatment with 50μM 3-(2-hydroxyl-1-methyl-2-nitrosohydrazino)-N-methyl-1-propanamine (interrupted line) or after exposure to CO (dotted line). post GF, post gel filtration; AU, arbitrary units.