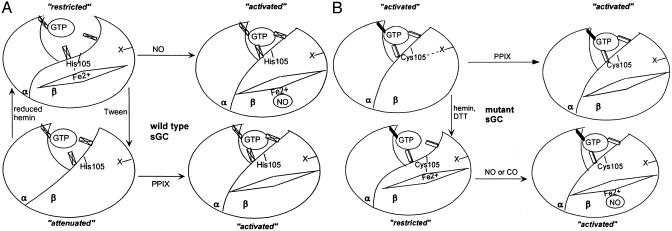
Fig. 6.
Schematic representation of heme-dependent regulation of wild type (A) and αβCys-105 mutant (B). Catalytic center is schematically represented as a GTP molecule interacting with three structural elements. Maximal catalytic activity is achieved when all three elements are firmly interacting with GTP: activated conformation. Insertion of the heme and formation of a coordinating bond with His-105 (wild type) or Cys-105 (mutant) induces changes in the regulatory domain, affects the optimal structure of catalytic center, and results in a restricted or inhibited conformation. Activated conformation can be achieved by binding of NO to the heme moiety and disruption of the heme-coordinating bond. Removal of heme in wild-type enzyme results in an intermediate attenuated conformation, which in theαβCys-105 mutant mimics activated conformation due to a stabilizing effect of a DTT-sensitive modification or interaction designated as “X.” Binding of PPIX to a heme-deficient enzyme (wild type or mutant) also results in activated conformation.