Abstract
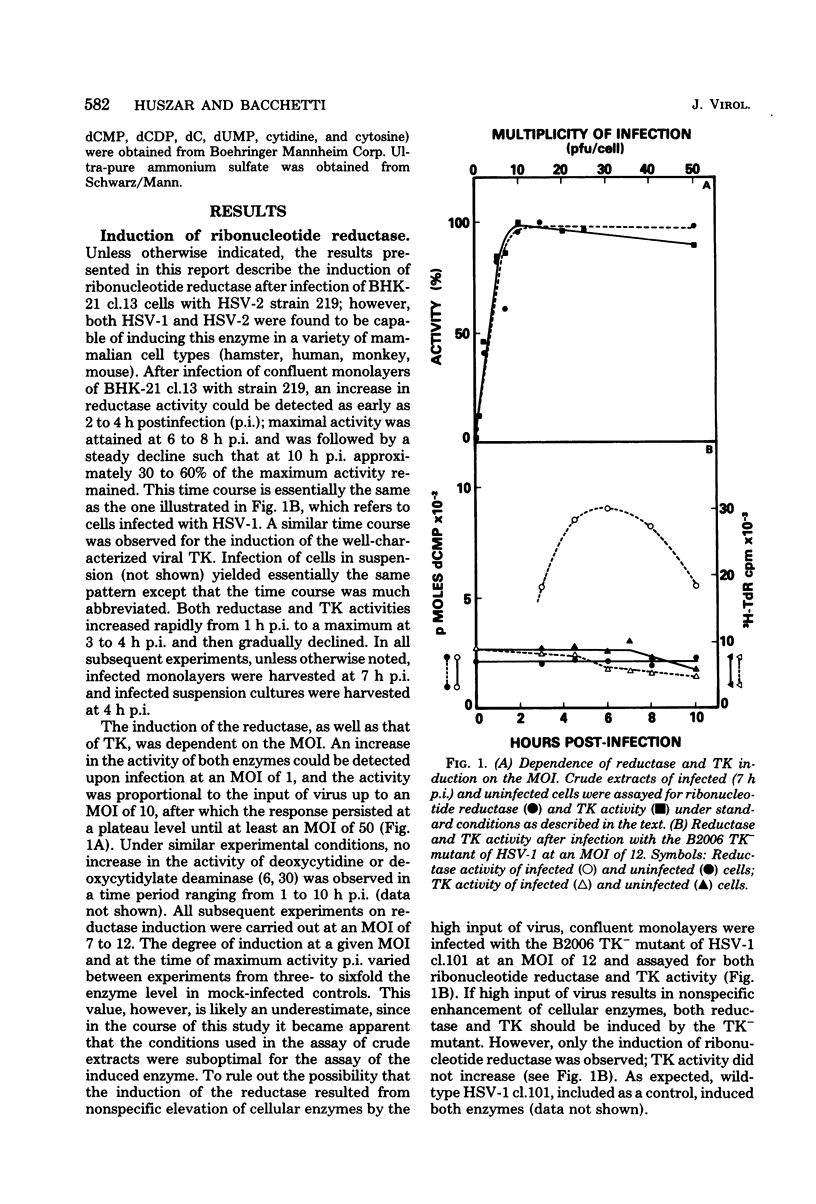
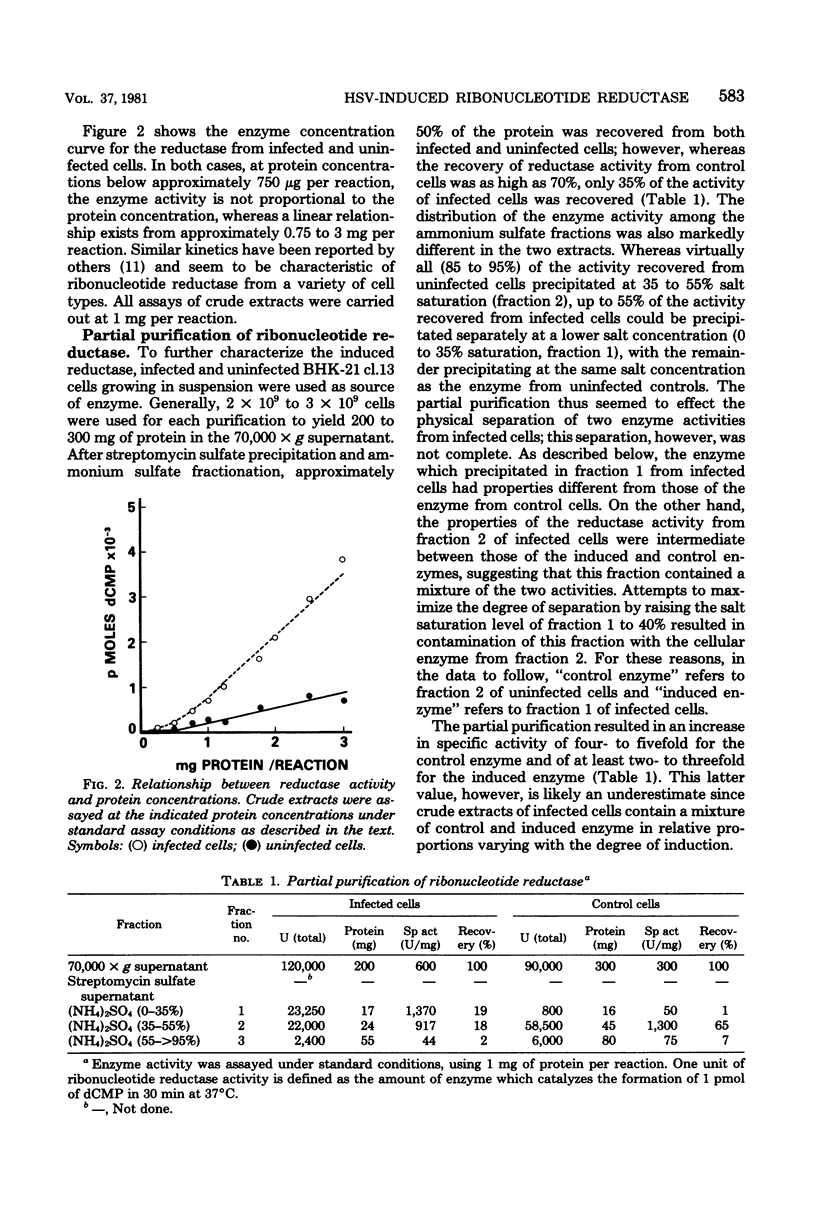
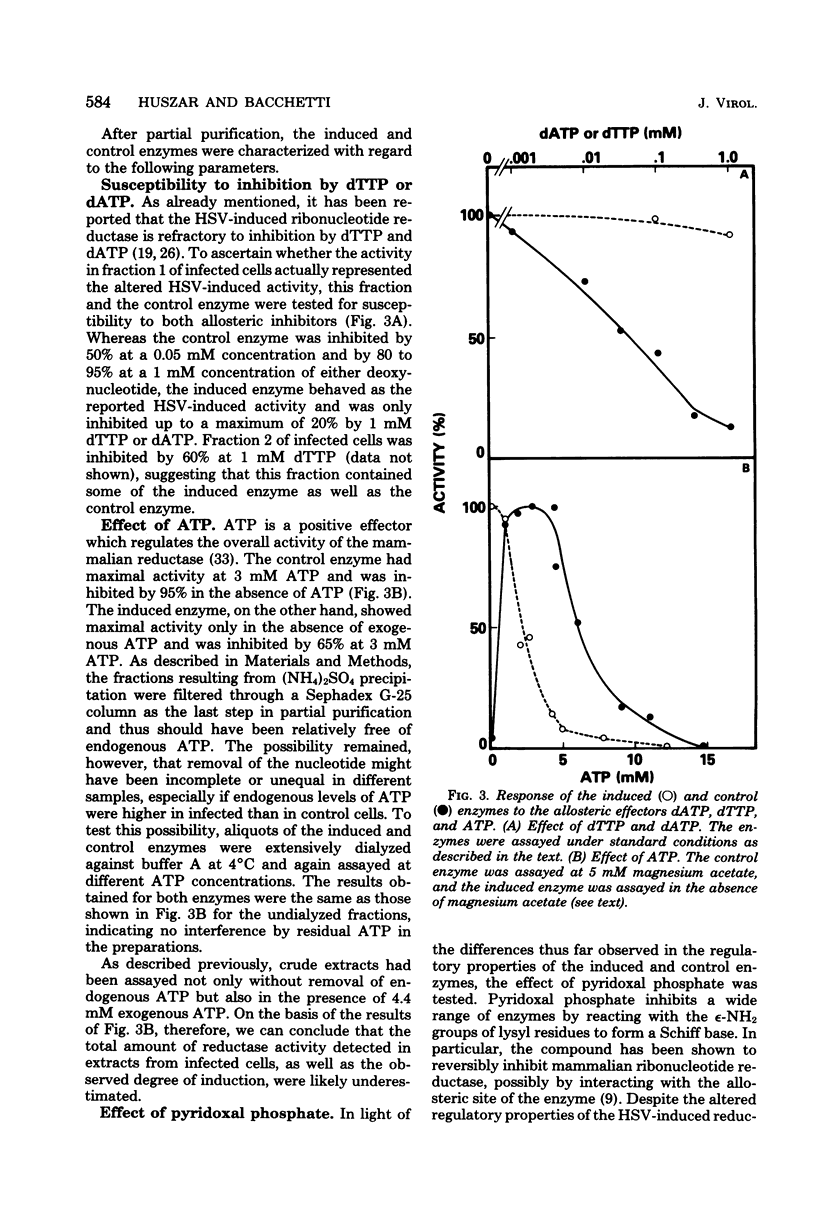
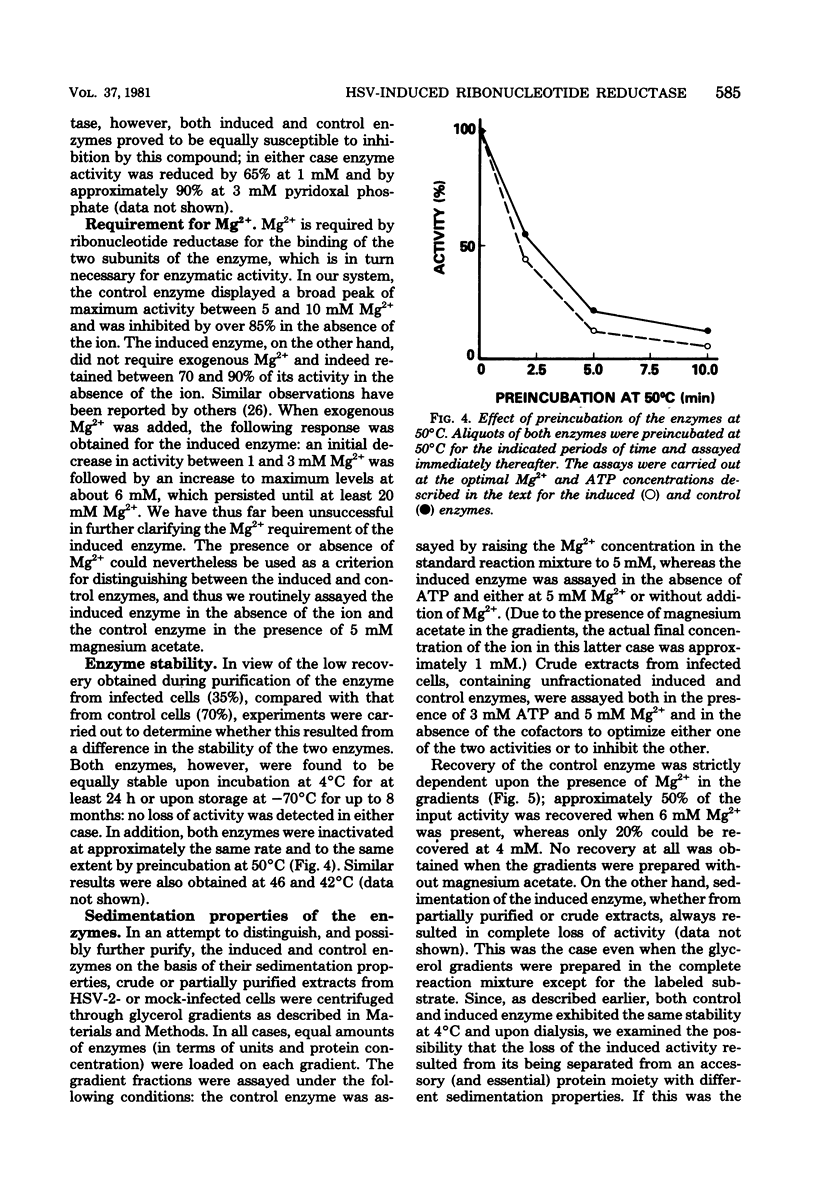
In this report we confirm and further characterize the induction of a novel ribonucleotide reductase after herpes simplex virus infection of mammalian cells. Induction of the enzyme was observed at a multiplicity of infection of 1 PFU/cell or greater and was found to be maximal (three- to sixfold the activity in mock-infected controls at 6 to 8 h postinfection at a multiplicity of infection of 10 PFU/cell. Partial purification and subsequent characterization of the reductase activity from infected cells demonstrated the existence of two enzymes which could be separated by precipitation with ammonium sulfate. One of the activities precipitated at between 35 and 55% salt saturation, as did the enzyme from control cells, whereas the novel activity precipitated at 0 to 35% saturation. This latter enzyme was similar to the herpes simplex virus-induced reductase described by others in its lack of requirement for Mg2 and its resistance to inhibition by dTTP and dATP; in addition, we found that it was inhibited by ATP, whereas the enzyme from control cells displayed an absolute requirement for the nucleotide. Both enzymes were equally inhibited by pyridoxal phosphate and showed similar cold and heat stability. The enzyme induced by herpes simplex virus infection, however, was much more labile than the control enzyme upon purification.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K., Rhodes D. P. In vitro synthesis of RNA that contains polyadenylate by virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3566–3570. doi: 10.1073/pnas.70.12.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund O., Karlström O., Reichard P. A new ribonucleotide reductase system after infection with phage T4. Proc Natl Acad Sci U S A. 1969 Mar;62(3):829–835. doi: 10.1073/pnas.62.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund O. Ribonucleoside diphosphate reductase induced by bacteriophage T4. II. Allosteric regulation of substrate sepecificity and catalytic activity. J Biol Chem. 1972 Nov 25;247(22):7276–7281. [PubMed] [Google Scholar]
- Berglund O. Ribonucleoside diphosphate reductase induced by bacteriophage Tr. I. Purification and characterization. J Biol Chem. 1972 Nov 25;247(22):7270–7275. [PubMed] [Google Scholar]
- Chan T. S. Induction of deoxycytidine deaminase activity in mammalian cell lines by infection with herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1734–1738. doi: 10.1073/pnas.74.4.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Goz B., Prusoff W. H. Deoxyribonucleotide metabolism in Herpes simplex virus infected HeLa cells. Biochim Biophys Acta. 1975 May 16;390(3):253–263. doi: 10.1016/0005-2787(75)90346-9. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Henry B. E., Randall C. C., O'Callaghan D. J. Ribonucleotide reductase activity in hydroxyurea-resistant herpesvirus replication. Proc Soc Exp Biol Med. 1977 Jul;155(3):395–399. doi: 10.3181/00379727-155-39815. [DOI] [PubMed] [Google Scholar]
- Cory J. G., Mansell M. M. Studies on mammalian ribonucleotide reductase inhibition by pyridoxal phosphate and the dialdehyde derivatives of adenosine, adenosine 5'-monophosphate, and adenosine 5'-triphosphate. Cancer Res. 1975 Feb;35(2):390–396. [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Thelander L., Akerman M. Ribonucleotide reductase from calf thymus. Purification and properties. Biochemistry. 1979 Jul 10;18(14):2941–2948. doi: 10.1021/bi00581a004. [DOI] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Henry B. E., Glaser R., Hewetson J., O'Callaghan D. J. Expression of altered ribonucleotide reductase activity associated with the replication of the Epstein-Barr virus. Virology. 1978 Aug;89(1):262–271. doi: 10.1016/0042-6822(78)90058-2. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Bjursell G. Deoxyribonucleoside triphosphate pools in cells infected with deoxypyrimidine kinaseless herpes simplex virus. J Gen Virol. 1976 Apr;31(1):115–123. doi: 10.1099/0022-1317-31-1-115. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Bjursell G. Deoxyribonucleoside triphosphate pools in herpes simplex type 1 infected cells. J Gen Virol. 1976 Apr;31(1):101–113. doi: 10.1099/0022-1317-31-1-101. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Klemperer H. G., Haynes G. R., Shedden W. I., Watson D. H. A virus-specific thymidine kinase in BHK-21 cells infected with herpes simplex virus. Virology. 1967 Jan;31(1):120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langelier Y., Déchamps M., Buttin G. Aanlysis of dCMP deaminase and CDP reductase levels in hamster cells infected by herpes simplex virus. J Virol. 1978 Jun;26(3):547–553. doi: 10.1128/jvi.26.3.547-553.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Nordenskjöld B. A., Skoog L., Brown N. C., Reichard P. Deoxyribonucleotide pools and deoxyribonucleic acid synthesis in cultured mouse embryo cells. J Biol Chem. 1970 Oct 25;245(20):5360–5368. [PubMed] [Google Scholar]
- Peterson D. M., Moore E. C. Independent fluctuations of cytidine and adenosine diphosphate reductase activities in cultured Chinese hamster fibroblasts. Biochim Biophys Acta. 1976 Apr 15;432(1):80–91. doi: 10.1016/0005-2787(76)90043-5. [DOI] [PubMed] [Google Scholar]
- Ponce de Leon M., Eisenberg R. J., Cohen G. H. Ribonucleotide reductase from herpes simplex virus (types 1 and 2) infected and uninfected KB cells: properties of the partially purified enzymes. J Gen Virol. 1977 Jul;36(1):163–173. doi: 10.1099/0022-1317-36-1-163. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Lewis R. B., Powell K. L. Identification of the herpes simplex virus DNA polymerase gene. Nature. 1977 Oct 13;269(5629):621–623. doi: 10.1038/269621a0. [DOI] [PubMed] [Google Scholar]
- Rolton H. A., Keir H. M. Deoxycytidylate deaminase evidence for a new enzyme in cells infected by the virus of herpes simplex. Biochem J. 1974 Nov;143(2):403–409. doi: 10.1042/bj1430403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Rawls W. E., Duff R., Rapp F., Adam E., Melnick J. L. Antigenic differences between isolates of herpesvirus type 2. Intervirology. 1974;3(1-2):1–14. doi: 10.1159/000149738. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]


