Abstract
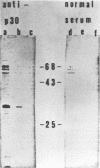
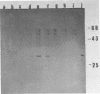
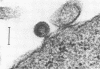
In contrast to those of many other mouse strains, spleen cell cultures of 129/J mice do not release reverse transcriptase activity into the supernatant upon stimulation with bacterial lipopolysaccharide. We report here that lipopolysaccharide induced the expression of intracellular viral proteins in 129/J spleen cells. Furthermore, we found that stimulated spleen cells released retroviral particles. We conclude that 129/J mice are inducible with lipopolysaccharide but that the virus produced is a defective particle deficient in reverse transcriptase activity.
Full text
PDF

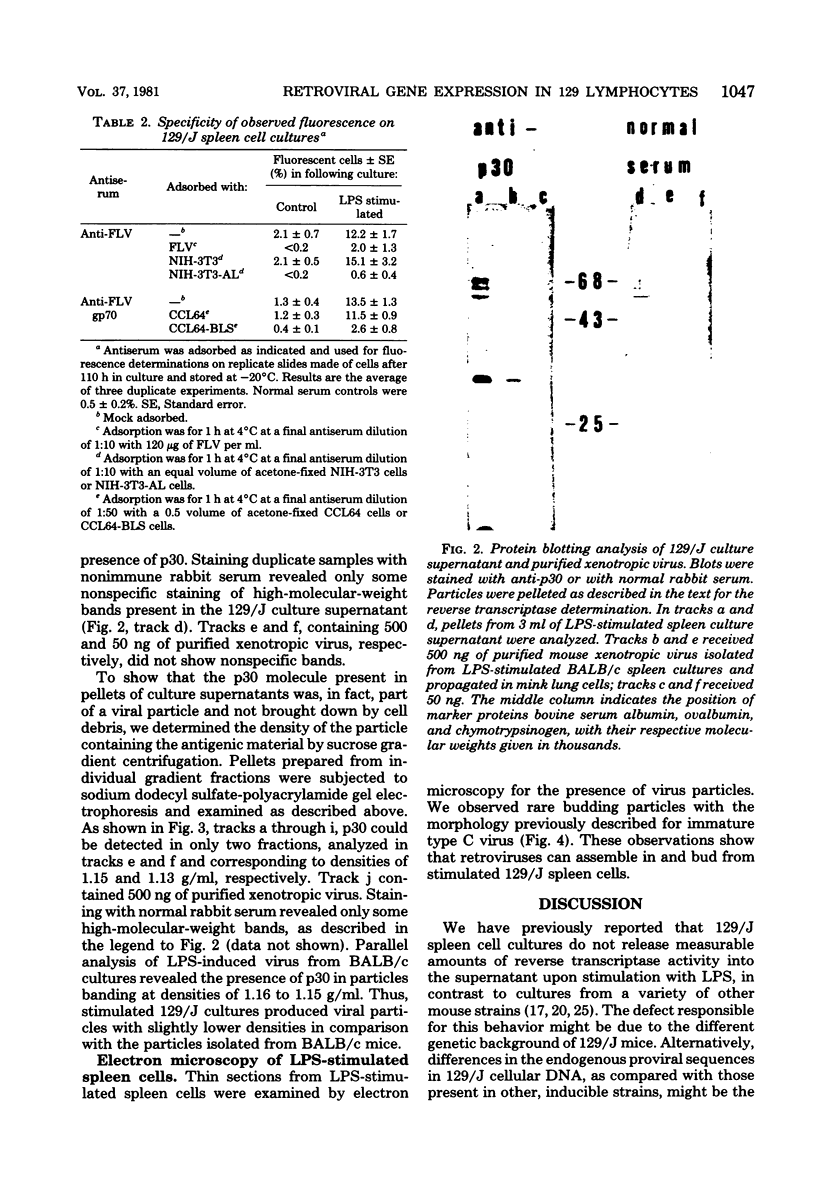

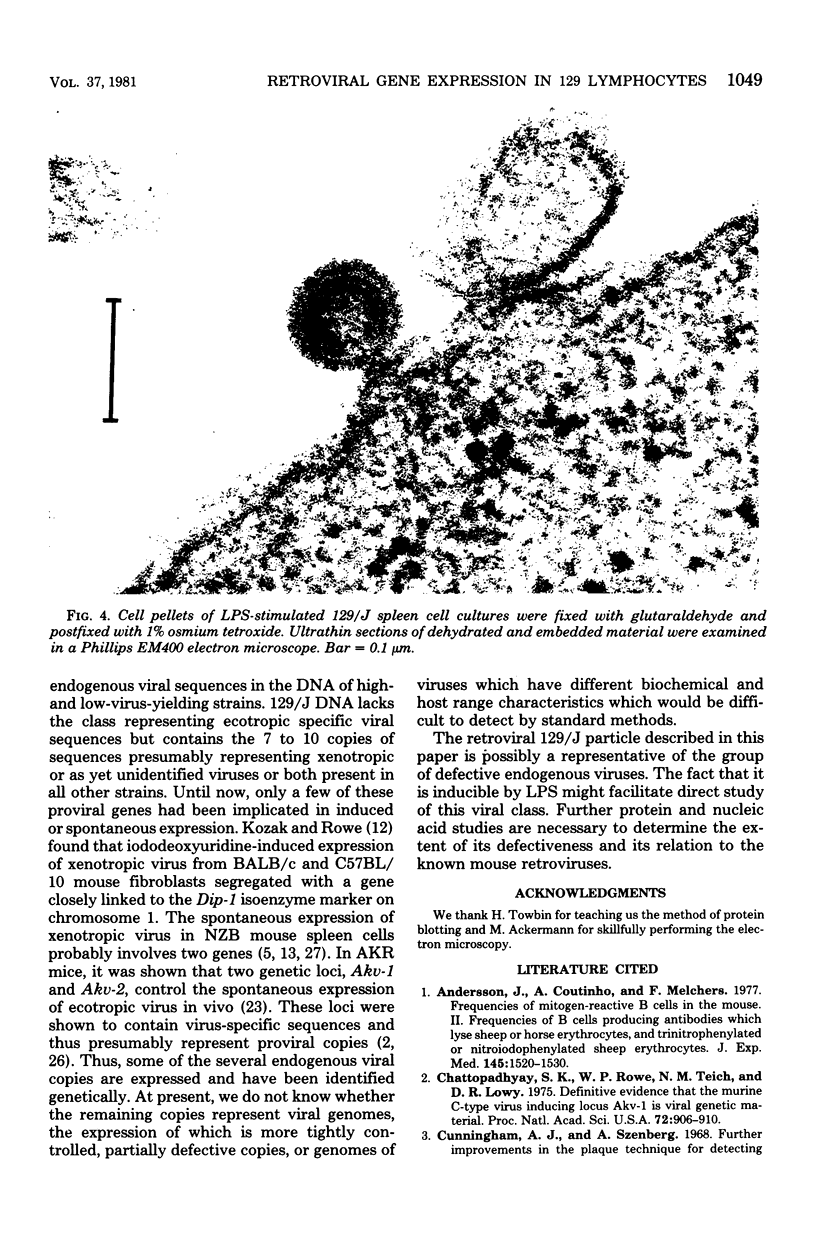

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Melchers F. Frequencies of mitogen-reactive B cells in the mouse. II. Frequencies of B cells producing antibodies which lyse sheep or horse erythrocytes, and trinitrophenylated or nitroiodophenylated sheep erythrocytes. J Exp Med. 1977 Jun 1;145(6):1520–1530. doi: 10.1084/jem.145.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Rowe W. P., Teich N. M., Lowy D. R. Definitive evidence that the murine C-type virus inducing locus Akv-1 is viral genetic material. Proc Natl Acad Sci U S A. 1975 Mar;72(3):906–910. doi: 10.1073/pnas.72.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Tronick S. R., Aaronson S. A. Immunological relationships of an endogenous guinea pig retrovirus with prototype mammalian type B and type D retroviruses. J Virol. 1980 Jan;33(1):522–530. doi: 10.1128/jvi.33.1.522-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. K., Schwartz R. S. Genetics of expression of xenotropic virus and autoimmunity in NZB mice. Nature. 1976 Sep 30;263(5576):412–415. doi: 10.1038/263412b0. [DOI] [PubMed] [Google Scholar]
- Forni L., Coutinho A. Receptor interactions on the membrane of resting and activated B cells. Nature. 1978 May 25;273(5660):304–306. doi: 10.1038/273304a0. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Phillips S. M., Stephenson J. R., Aaronson S. A. Induction of mouse type-C RNA virus by lipopolysaccharide. J Immunol. 1975 Jul;115(1):317–320. [PubMed] [Google Scholar]
- Hilgers J., Nowinski R. C., Geering G., Hardy W. Detection of avian and mammalian oncogenic RNA viruses (oncornaviruses) by immunofluorescence. Cancer Res. 1972 Jan;32(1):98–106. [PubMed] [Google Scholar]
- Hunsmann G., Moennig V., Pister L., Seifert E., Schäfer W. Properties of mouse leukemia viruses. VIII. The major viral glycoprotein of Friend leukemia virus. Seroimmunological, interfering and hemagglutinating capacities. Virology. 1974 Dec;62(2):307–318. doi: 10.1016/0042-6822(74)90394-8. [DOI] [PubMed] [Google Scholar]
- Kozak C., Rowe W. P. Genetic mapping of xenotropic leukemia virus-inducing loci in two mouse strains. Science. 1978 Mar 31;199(4336):1448–1449. doi: 10.1126/science.204014. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Joyner J., Nayar K. T., Kouri R. E. Genetics of xenotropic virus expression in mice. I. Evidence for a single locus regulating spontaneous production of infectious virus in crosses involving NZB/B1NJ and 129/J strains of mice. J Virol. 1979 Jun;30(3):754–758. doi: 10.1128/jvi.30.3.754-758.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Chattopadhyay S. K., Teich N. M., Rowe W. P., Levine A. S. AKR murine leukemia virus genome: frequency of sequences in DNA of high-, low-, and non-virus-yielding mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3555–3559. doi: 10.1073/pnas.71.9.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock P. R., Ihle J. N., Joseph D. R. Expression of AKR murine leukemia virus gp71-like and BALB(X) gp-71-like antigens in normal mouse tissues in the absence of overt virus expression. J Exp Med. 1977 Aug 1;146(2):422–434. doi: 10.1084/jem.146.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monckton R. P., Moroni C. Foetal calf serum acts as an inducer of endogenous C-type virus in mouse lymphoid cells. J Gen Virol. 1980 Mar;47(1):59–66. doi: 10.1099/0022-1317-47-1-59. [DOI] [PubMed] [Google Scholar]
- Moroni C., Schumann G. Mitogen induction of murine C-type viruses. II. Effect of B-lymphocyte mitogens. Virology. 1976 Aug;73(1):17–22. doi: 10.1016/0042-6822(76)90056-8. [DOI] [PubMed] [Google Scholar]
- Moroni C., Schumann G. Mitogen induction of murine C-type viruses. IV. Effects of lipoprotein E. coli, pokeweed mitogen and dextran sulphate. J Gen Virol. 1978 Mar;38(3):497–503. doi: 10.1099/0022-1317-38-3-497. [DOI] [PubMed] [Google Scholar]
- Moroni C., Schumann G., Robert-Guroff M., Suter E. R., Martin D. Induction of endogenous murine C-type virus in spleen cell cultures treated with mitogens and 5-bromo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1975 Feb;72(2):535–538. doi: 10.1073/pnas.72.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Fan H. Monospecific immunoprecipitation of murine leukemia virus polyribosomes: identification of p30 protein-specific messenger RNA. Cell. 1976 Dec;9(4 Pt 1):579–588. doi: 10.1016/0092-8674(76)90040-4. [DOI] [PubMed] [Google Scholar]
- Phillips S. M., Stephenson J. R., Aaronson S. A. Genetic factors infuencing mouse type-C RNA virus induction by naturally occurring B cell mitogens. J Immunol. 1977 Feb;118(2):662–666. [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G., Moroni C. Mitogen induction of murine C-type viruses. I. Analysis of lymphoid cell subpopulations. J Immunol. 1976 Apr;116(4):1145–1150. [PubMed] [Google Scholar]
- Schumann G., Moroni C. Mitogen induction of murine C-type viruses. III. Effect of culture conditions, age, and genotype. Virology. 1977 Jun 1;79(1):81–87. doi: 10.1016/0042-6822(77)90336-1. [DOI] [PubMed] [Google Scholar]
- Steffen D., Bird S., Rowe W. P., Weinberg R. A. Identification of DNA fragments carrying ecotropic proviruses of AKR mice. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4554–4558. doi: 10.1073/pnas.76.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Demonstration of a genetic factor influencing spontaneous release of a xenotropic virus of mouse cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4925–4929. doi: 10.1073/pnas.71.12.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., Old L. J., Boyse E. A. The G-IX system. A cell surface allo-antigen associated with murine leukemia virus; implications regarding chromosomal integration of the viral genome. J Exp Med. 1971 Jun 1;133(6):1334–1355. doi: 10.1084/jem.133.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., Lilly F., August J. T. Host control of endogenous murine leukemia virus gene expression: concentrations of viral proteins in high and low leukemia mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3682–3686. doi: 10.1073/pnas.71.9.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. S., Vitetta E. S., Fleissner E., Boyse E. A. Biochemical evidence linking the GIX thymocyte surface antigen to the gp69/71 envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):198–205. doi: 10.1084/jem.141.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]