Abstract
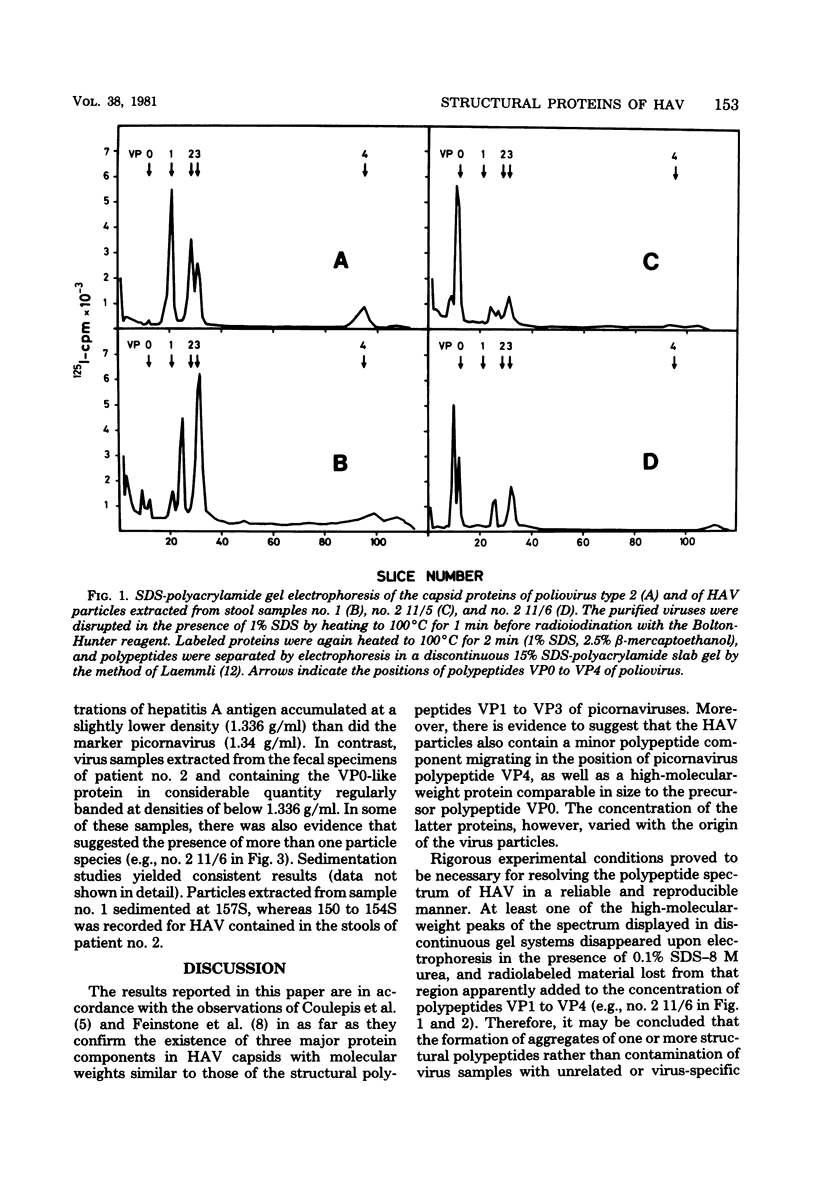
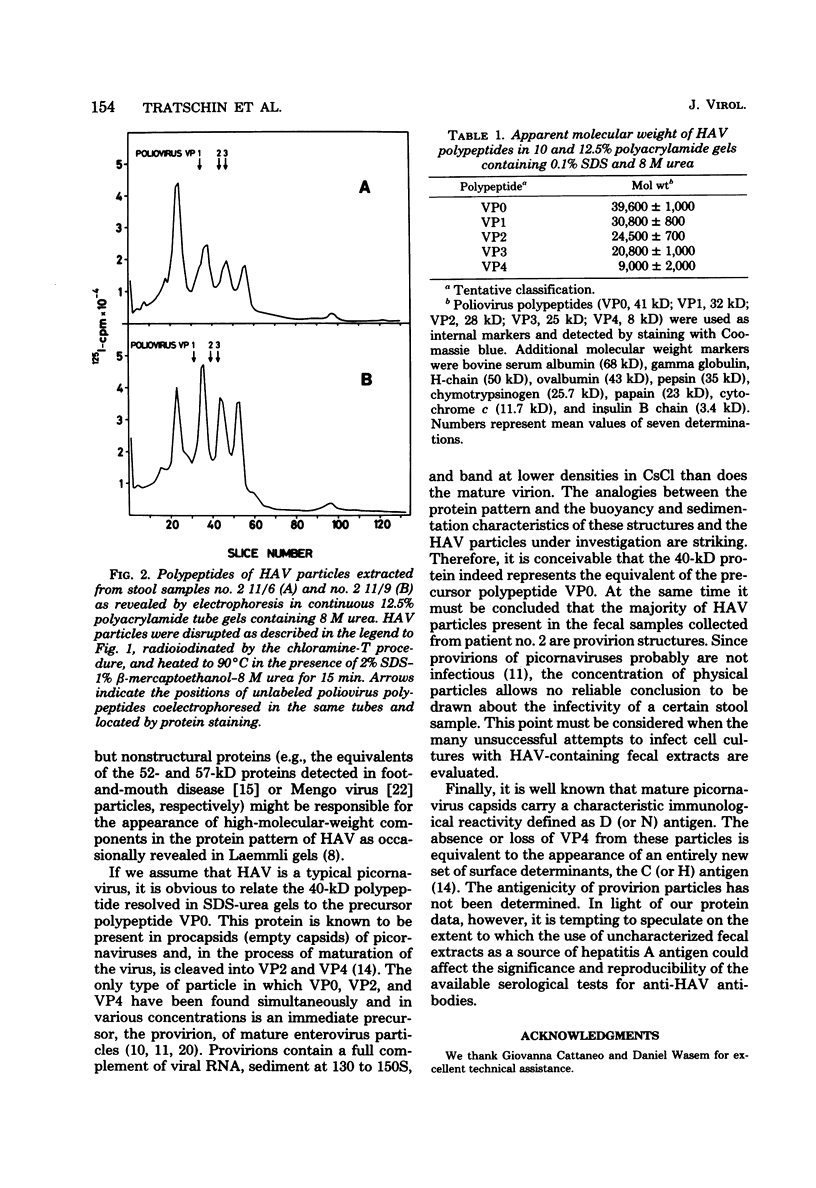
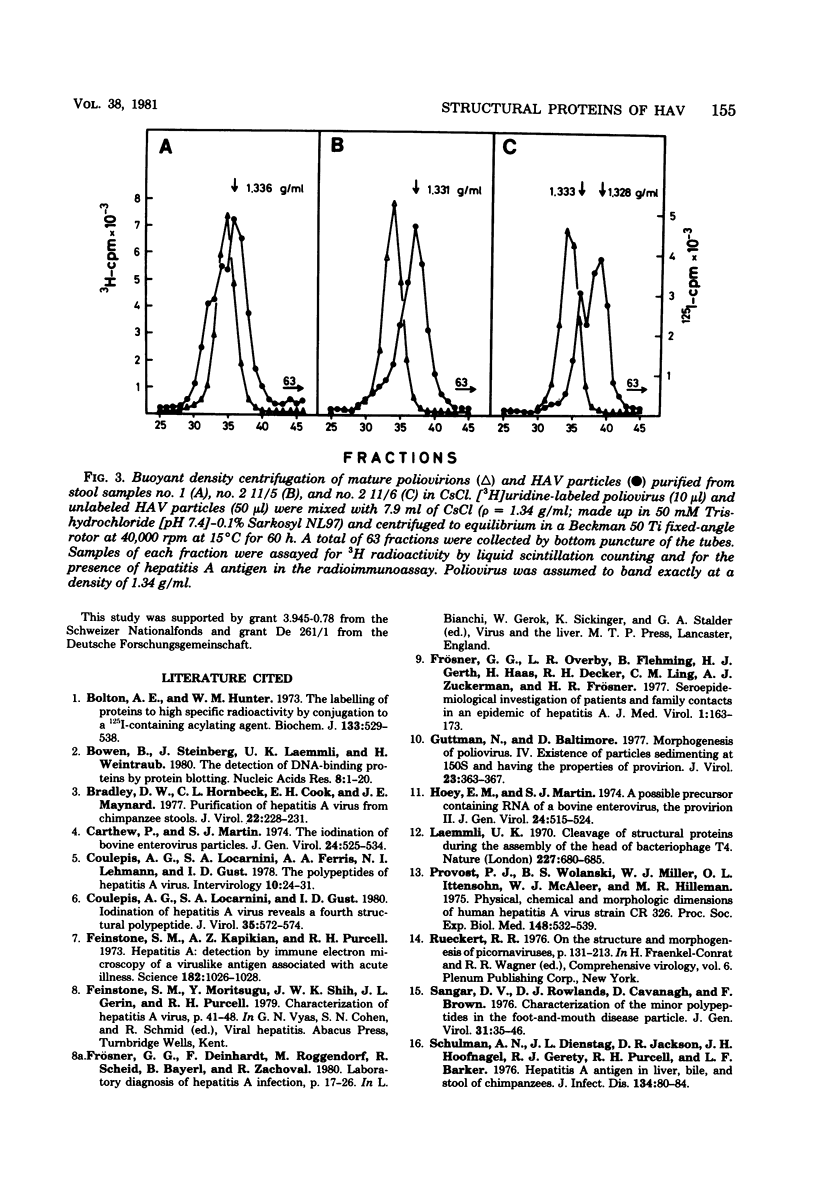
Hepatitis A virus was purified from fecal samples collected at various times in the incubation period of patients with naturally acquired hepatitis A. The proteins of particles banding at around 1.34 g/ml in CsCl and sedimenting at about 160S were radioiodinated in vitro and separated by electrophoresis on polyacrylamide gels in the presence of 0.1% sodium dodecyl sulfate and 8 M urea. Under these conditions, the capsid proteins resolved into four polypeptides with molecular weights of approximately 31,000, 24,500, 21,000, and 9,000, respectively. A fifth protein of about 40,000 daltons in size and assumed to be equivalent to the precursor polypeptide VP0 of the picornaviruses was present in particles sedimenting at only 150 to 155S and banding at around 1.33 g/ml in CsCl. The physicochemical characteristics of these particles are consistent with those of the provirion structures of picornaviruses. In several of the fecal samples, these particles represented a considerable fraction of all particles present. The significance of this finding with respect to the antigenicity of hepatitis A antigen extracted from stool specimens is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. W., Hornbeck C. L., Cook E. H., Maynard J. E. Purification of hepatitis A virus from chimpanzee stools. J Virol. 1977 Apr;22(1):228–231. doi: 10.1128/jvi.22.1.228-231.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew P., Martin S. J. The iodination of bovine enterovirus particles. J Gen Virol. 1974 Sep;24(3):525–534. doi: 10.1099/0022-1317-24-3-525. [DOI] [PubMed] [Google Scholar]
- Coulepis A. G., Locarnini S. A., Ferris A. A., Lehmann N. I., Gust I. D. The polypeptides of hepatitis A virus. Intervirology. 1978;10(1):24–31. doi: 10.1159/000148964. [DOI] [PubMed] [Google Scholar]
- Coulepis A. G., Locarnini S. A., Gust I. D. Iodination of hepatitis A virus reveals a fourth structural polypeptide. J Virol. 1980 Aug;35(2):572–574. doi: 10.1128/jvi.35.2.572-574.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstone S. M., Kapikian A. Z., Purceli R. H. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973 Dec 7;182(4116):1026–1028. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- Frösner G. G., Overby L. R., Flehmig B., Gerth H. J., Haas H., Decker R. H., Ling C. M., Zuckerman A. J., Frösner H. R. Seroepidemiological investigation of patients and family contacts in an epidemic of hepatitis A. J Med Virol. 1977;1(3):163–173. doi: 10.1002/jmv.1890010303. [DOI] [PubMed] [Google Scholar]
- Guttman N., Baltimore D. Morphogenesis of poliovirus. IV. existence of particles sedimenting at 150S and having the properties of provirion. J Virol. 1977 Aug;23(2):363–367. doi: 10.1128/jvi.23.2.363-367.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey E. M., Martin S. J. A possible precursor containing RNA of a bovine enterovirus: the provirion 11. J Gen Virol. 1974 Sep;24(3):515–524. doi: 10.1099/0022-1317-24-3-515. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Wolanski B. S., Miller W. J., Ittensohn O. L., McAleer W. J., Hilleman M. R. Physical, chemical and morphologic dimensions of human hepatitis A virus strain CR326 (38578). Proc Soc Exp Biol Med. 1975 Feb;148(2):532–539. doi: 10.3181/00379727-148-38578. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Rowlands D. J., Cavanagh D., Brown F. Characterization of the minor polypeptides in the foot-and-mouth disease particle. J Gen Virol. 1976 Apr;31(1):35–46. doi: 10.1099/0022-1317-31-1-35. [DOI] [PubMed] [Google Scholar]
- Schulman A. N., Dienstag J. L., Jackson D. R., Hoofnagle J. H., Gerety R. J., Purcell R. H., Barker L. F. Hepatitis A antigen particles in liver, bile, and stool of chimpanzees. J Infect Dis. 1976 Jul;134(1):80–84. doi: 10.1093/infdis/134.1.80. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. K., Mathiesen L. R., Lorenz D., Drucker J., Feinstone S. M., Wagner J. A., Purcell R. H. Localization of hepatitis A antigen in liver tissue by peroxidase-conjugated antibody method: light and electron microscopic studies. J Immunol. 1978 Nov;121(5):1671–1679. [PubMed] [Google Scholar]
- Siegl G., Frösner G. G. Characterization and classification of virus particles associated with hepatitis A. I. Size, density, and sedimentation. J Virol. 1978 Apr;26(1):40–47. doi: 10.1128/jvi.26.1.40-47.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Frösner G. G. Characterization and classification of virus particles associated with hepatitis A. II. Type and configuration of nucleic acid. J Virol. 1978 Apr;26(1):48–53. doi: 10.1128/jvi.26.1.48-53.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. T., Taylor M. W. Morphogenesis of picornaviruses: characterization and assembly of bovine enterovirus subviral particles. J Gen Virol. 1976 Mar;30(3):317–328. doi: 10.1099/0022-1317-30-3-317. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. I. Polypeptide and ribonucleate components of the virus particle. Virology. 1974 Feb;57(2):531–542. doi: 10.1016/0042-6822(74)90192-5. [DOI] [PubMed] [Google Scholar]


