Abstract
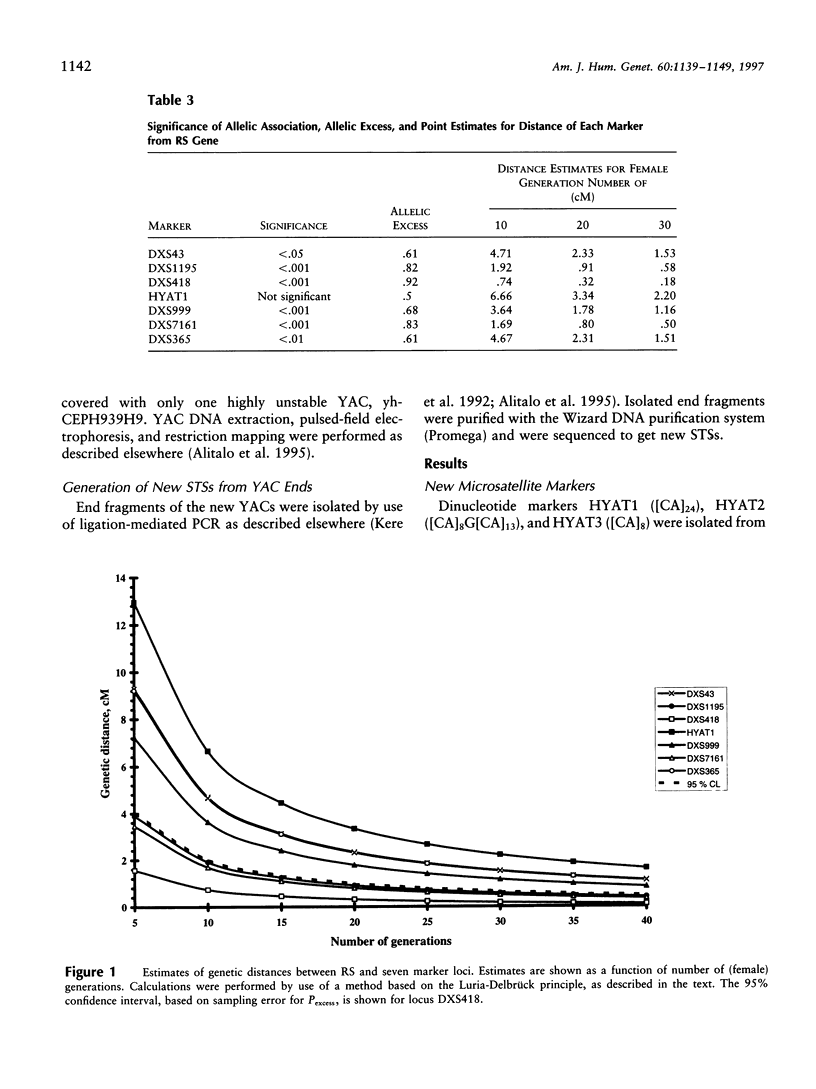
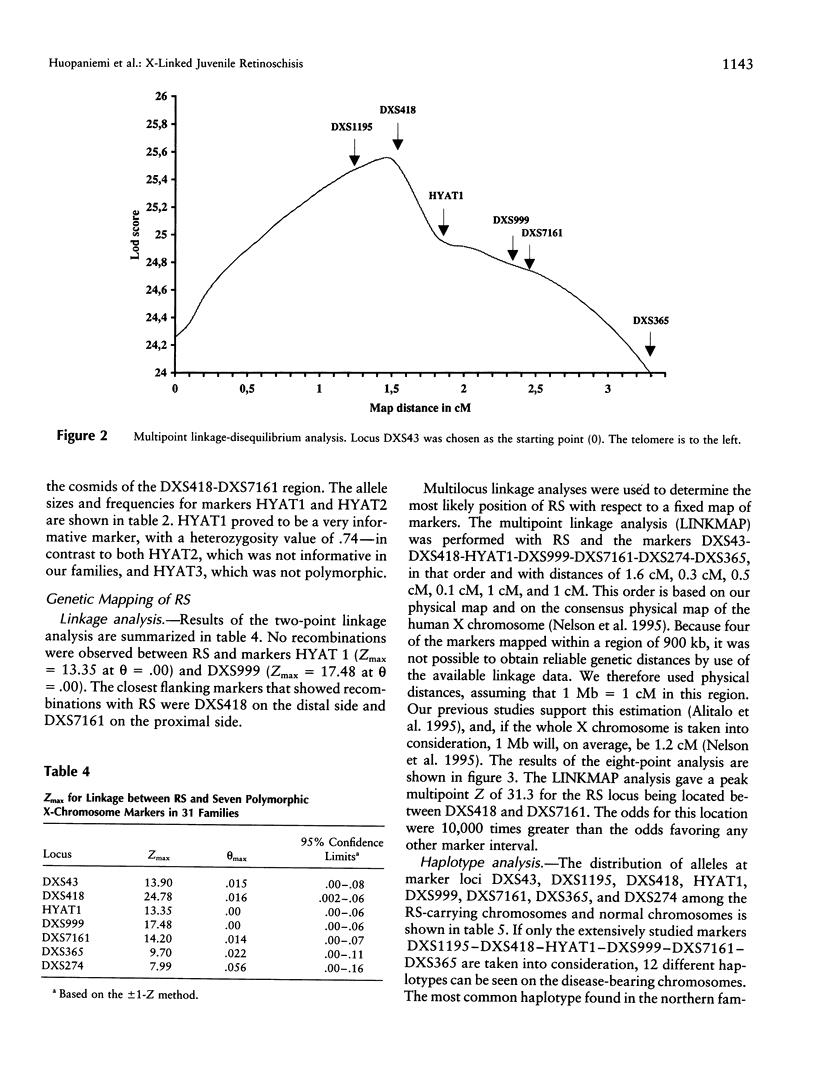
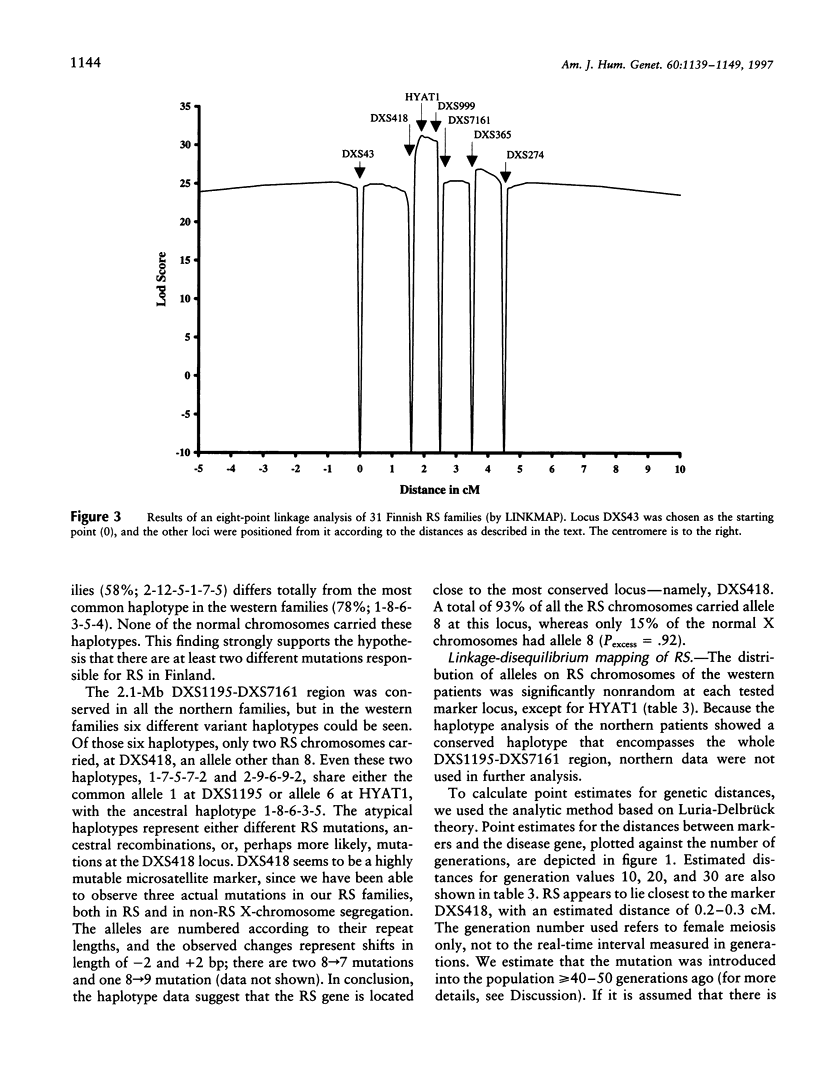
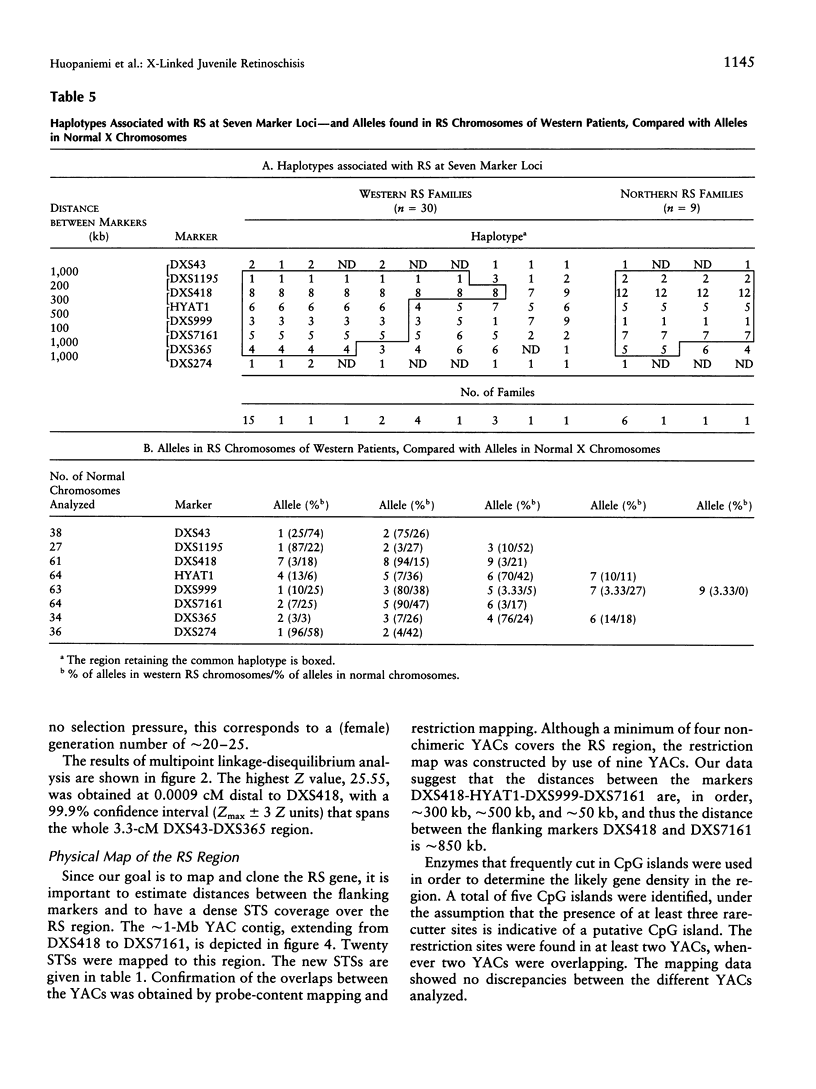
X-linked juvenile retinoschisis (RS) is a recessively inherited disorder resulting in poor visual acuity. Affected males typically show retinal degeneration and intraretinal splitting. The prevalence of RS is 1:15,000-1:30,000. Elsewhere we have mapped the RS gene between the markers DXS43 and DXS274 in Xp22.1-p22.2. To narrow the RS region, we analyzed 31 Finnish RS families with the markers DXS418, DXS999, DXS7161, and DXS365 and a new polymorphic microsatellite marker, HYAT1. Multipoint linkage analysis allowed us to localize the RS gene between the markers DXS418 and DXS7161 (LOD score = 31.3). We have covered this region with nine YAC clones. On the basis of the sizes of the YACs, sequence-tagged site (STS) content mapping, and restriction mapping, the physical distance between DXS418 and DXS7161 is approximately 0.9 Mb. A total of five potential CpG islands could be identified. For haplotype analysis, eight additional Finnish RS families were analyzed with the markers DXS1195, DXS418, HYAT1, DXS999, DXS7161, and DXS365. On the basis of the linkage-disequilibrium data that were derived from the genetically isolated Finnish population, the critical region for RS could be narrowed to 0.2-0.3 cM, between the markers DXS418 and HYAT1.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsen H. M., Abderrahim H., Cann H. M., Dausset J., Le Paslier D., Cohen D. Construction and characterization of a yeast artificial chromosome library containing seven haploid human genome equivalents. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4256–4260. doi: 10.1073/pnas.87.11.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo T., Forsius H., Kärnä J., Frants R. R., Eriksson A. W., Wood S., Kruse T. A., de la Chapelle A. Linkage relationships and gene order around the locus for X-linked retinoschisis. Am J Hum Genet. 1988 Oct;43(4):476–483. [PMC free article] [PubMed] [Google Scholar]
- Alitalo T., Francis F., Kere J., Lehrach H., Schlessinger D., Willard H. F. A 6-Mb YAC contig in Xp22.1-p22.2 spanning the DXS69E, XE59, GLRA2, PIGA, GRPR, CALB3, and PHKA2 genes. Genomics. 1995 Feb 10;25(3):691–700. doi: 10.1016/0888-7543(95)80012-b. [DOI] [PubMed] [Google Scholar]
- Alitalo T., Kruse T. A., de la Chapelle A. Refined localization of the gene causing X-linked juvenile retinoschisis. Genomics. 1991 Mar;9(3):505–510. doi: 10.1016/0888-7543(91)90417-d. [DOI] [PubMed] [Google Scholar]
- Alitalo T., Kärnä J., Forsius H., de la Chapelle A. X-linked retinoschisis is closely linked to DXS41 and DXS16 but not DXS85. Clin Genet. 1987 Sep;32(3):192–195. doi: 10.1111/j.1399-0004.1987.tb03353.x. [DOI] [PubMed] [Google Scholar]
- Arden G. B., Gorin M. B., Polkinghorne P. J., Jay M., Bird A. C. Detection of the carrier state of X-linked retinoschisis. Am J Ophthalmol. 1988 Jun 15;105(6):590–595. doi: 10.1016/0002-9394(88)90049-9. [DOI] [PubMed] [Google Scholar]
- Bassam B. J., Caetano-Anollés G., Gresshoff P. M. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem. 1991 Jul;196(1):80–83. doi: 10.1016/0003-2697(91)90120-i. [DOI] [PubMed] [Google Scholar]
- Bergen A. A., ten Brink J. B., Bleeker-Wagemakers L. M., van Schooneveld M. J. Refinement of the chromosomal position of the X linked juvenile retinoschisis gene. J Med Genet. 1994 Dec;31(12):972–975. doi: 10.1136/jmg.31.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi G. The isochore organization of the human genome. Annu Rev Genet. 1989;23:637–661. doi: 10.1146/annurev.ge.23.120189.003225. [DOI] [PubMed] [Google Scholar]
- Browne D., Barker D., Litt M. Dinucleotide repeat polymorphisms at the DXS365, DXS443 and DXS451 loci. Hum Mol Genet. 1992 Jun;1(3):213–213. doi: 10.1093/hmg/1.3.213. [DOI] [PubMed] [Google Scholar]
- Condon G. P., Brownstein S., Wang N. S., Kearns J. A., Ewing C. C. Congenital hereditary (juvenile X-linked) retinoschisis. Histopathologic and ultrastructural findings in three eyes. Arch Ophthalmol. 1986 Apr;104(4):576–583. doi: 10.1001/archopht.1986.01050160132029. [DOI] [PubMed] [Google Scholar]
- Cottingham R. W., Jr, Idury R. M., Schäffer A. A. Faster sequential genetic linkage computations. Am J Hum Genet. 1993 Jul;53(1):252–263. [PMC free article] [PubMed] [Google Scholar]
- Dahl N., Goonewardena P., Chotai J., Anvret M., Pettersson U. DNA linkage analysis of X-linked retinoschisis. Hum Genet. 1988 Mar;78(3):228–232. doi: 10.1007/BF00291666. [DOI] [PubMed] [Google Scholar]
- Dumur V., Trivier E., Puech B., Peugnet F., Zanlonghi X., Hache J. C., Hanauer A. Genetic analysis of new French X-linked juvenile retinoschisis kindreds using microsatellite markers closely linked to the RS locus: further narrowing of the RS candidate region. Hum Genet. 1995 Jul;96(1):79–82. doi: 10.1007/BF00214190. [DOI] [PubMed] [Google Scholar]
- Eriksson A. W., Forsius H., Vainio-Mattila B. X-kromosomaalinen retinoskiisi. Duodecim. 1972;88(1):43–51. [PubMed] [Google Scholar]
- Fasman K. H., Cuticchia A. J., Kingsbury D. T. The GDB Human Genome Data Base anno 1994. Nucleic Acids Res. 1994 Sep;22(17):3462–3469. doi: 10.1093/nar/22.17.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero G. B., Franco B., Roth E. J., Firulli B. A., Borsani G., Delmas-Mata J., Weissenbach J., Halley G., Schlessinger D., Chinault A. C. An integrated physical and genetic map of a 35 Mb region on chromosome Xp22.3-Xp21.3. Hum Mol Genet. 1995 Oct;4(10):1821–1827. doi: 10.1093/hmg/4.10.1821. [DOI] [PubMed] [Google Scholar]
- Gellert G., Peterson J., Krawczak M., Zoll B. Linkage relationship between retinoschisis and four marker loci. Hum Genet. 1988 Aug;79(4):382–384. doi: 10.1007/BF00282183. [DOI] [PubMed] [Google Scholar]
- Hästbacka J., de la Chapelle A., Kaitila I., Sistonen P., Weaver A., Lander E. Linkage disequilibrium mapping in isolated founder populations: diastrophic dysplasia in Finland. Nat Genet. 1992 Nov;2(3):204–211. doi: 10.1038/ng1192-204. [DOI] [PubMed] [Google Scholar]
- Hästbacka J., de la Chapelle A., Mahtani M. M., Clines G., Reeve-Daly M. P., Daly M., Hamilton B. A., Kusumi K., Trivedi B., Weaver A. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994 Sep 23;78(6):1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Höglund P., Sistonen P., Norio R., Holmberg C., Dimberg A., Gustavson K. H., de la Chapelle A., Kere J. Fine mapping of the congenital chloride diarrhea gene by linkage disequilibrium. Am J Hum Genet. 1995 Jul;57(1):95–102. [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Pelet A., Hentati H., Jeanpierre M., Briard M. L., Journel H., Munnich A., Dufier J. L. Contribution to carrier detection and genetic counselling in X linked retinoschisis. J Med Genet. 1991 Jun;28(6):383–388. doi: 10.1136/jmg.28.6.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kere J., Nagaraja R., Mumm S., Ciccodicola A., D'Urso M., Schlessinger D. Mapping human chromosomes by walking with sequence-tagged sites from end fragments of yeast artificial chromosome inserts. Genomics. 1992 Oct;14(2):241–248. doi: 10.1016/s0888-7543(05)80212-5. [DOI] [PubMed] [Google Scholar]
- Kidd K. K., Bowcock A. M., Schmidtke J., Track R. K., Ricciuti F., Hutchings G., Bale A., Pearson P., Willard H. F., Gelernter J. Report of the DNA committee and catalogs of cloned and mapped genes and DNA polymorphisms. Cytogenet Cell Genet. 1989;51(1-4):622–947. doi: 10.1159/000132810. [DOI] [PubMed] [Google Scholar]
- Larin Z., Monaco A. P., Lehrach H. Yeast artificial chromosome libraries containing large inserts from mouse and human DNA. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4123–4127. doi: 10.1073/pnas.88.10.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen F., Gundersen G., Lopez R., Prydz H. CpG islands as gene markers in the human genome. Genomics. 1992 Aug;13(4):1095–1107. doi: 10.1016/0888-7543(92)90024-m. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehesjoki A. E., Koskiniemi M., Norio R., Tirrito S., Sistonen P., Lander E., de la Chapelle A. Localization of the EPM1 gene for progressive myoclonus epilepsy on chromosome 21: linkage disequilibrium allows high resolution mapping. Hum Mol Genet. 1993 Aug;2(8):1229–1234. doi: 10.1093/hmg/2.8.1229. [DOI] [PubMed] [Google Scholar]
- Nagaraja R., Kere J., MacMillan S., Masisi M. J., Johnson D., Molini B. J., Halley G. R., Wein K., Trusgnich M., Eble B. Characterization of four human YAC libraries for clone size, chimerism and X chromosome sequence representation. Nucleic Acids Res. 1994 Aug 25;22(16):3406–3411. doi: 10.1093/nar/22.16.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevanlinna H. R. The Finnish population structure. A genetic and genealogical study. Hereditas. 1972;71(2):195–236. doi: 10.1111/j.1601-5223.1972.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Oudet C., Weber C., Kaplan J., Segues B., Croquette M. F., Roman E. O., Hanauer A. Characterisation of a highly polymorphic microsatellite at the DXS207 locus: confirmation of very close linkage to the retinoschisis disease gene. J Med Genet. 1993 Apr;30(4):300–303. doi: 10.1136/jmg.30.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar H., Bingham E. L., Lunetta K. L., Segal M., Richards J. E., Boehnke M., Sieving P. A. Refined genetic mapping of juvenile X-linked retinoschisis. Hum Hered. 1995 Jul-Aug;45(4):206–210. doi: 10.1159/000154290. [DOI] [PubMed] [Google Scholar]
- Pennacchio L. A., Lehesjoki A. E., Stone N. E., Willour V. L., Virtaneva K., Miao J., D'Amato E., Ramirez L., Faham M., Koskiniemi M. Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy (EPM1) Science. 1996 Mar 22;271(5256):1731–1734. doi: 10.1126/science.271.5256.1731. [DOI] [PubMed] [Google Scholar]
- Puech B., Kostrubiec B., Hache J. C., François P. Epidémiologie et prévalence des principales dystrophies rétiniennes héréditaires dans le Nord de la France. J Fr Ophtalmol. 1991;14(3):153–164. [PubMed] [Google Scholar]
- Sieving P. A., Bingham E. L., Roth M. S., Young M. R., Boehnke M., Kuo C. Y., Ginsburg D. Linkage relationship of X-linked juvenile retinoschisis with Xp22.1-p22.3 probes. Am J Hum Genet. 1990 Oct;47(4):616–621. [PMC free article] [PubMed] [Google Scholar]
- Sixth International Workshop on Human X Chromosome Mapping 1995. Banff, Alberta, Canada, June 16-18, 1995. Report and abstracts. Cytogenet Cell Genet. 1995;71(4):307–342. doi: 10.1159/000134135. [DOI] [PubMed] [Google Scholar]
- Sulisalo T., Klockars J., Mäkitie O., Francomano C. A., de la Chapelle A., Kaitila I., Sistonen P. High-resolution linkage-disequilibrium mapping of the cartilage-hair hypoplasia gene. Am J Hum Genet. 1994 Nov;55(5):937–945. [PMC free article] [PubMed] [Google Scholar]
- Tahvanainen E., Forsius H., Damsten M., Karila E., Kolehmainen J., Weissenbach J., Sistonen P., de la Chapelle A. Linkage disequilibrium mapping of the cornea plana congenita gene CNA2. Genomics. 1995 Dec 10;30(3):409–414. doi: 10.1006/geno.1995.1258. [DOI] [PubMed] [Google Scholar]
- Terwilliger J. D. A powerful likelihood method for the analysis of linkage disequilibrium between trait loci and one or more polymorphic marker loci. Am J Hum Genet. 1995 Mar;56(3):777–787. [PMC free article] [PubMed] [Google Scholar]
- Van De Vosse E., Booms P. F., Vossen R. H., Wapenaar M. C., Van Ommen G. J., Den Dunnen J. T. A CA-repeat polymorphism near DXS418 (P122). Hum Mol Genet. 1993 Dec;2(12):2202–2202. doi: 10.1093/hmg/2.12.2202-a. [DOI] [PubMed] [Google Scholar]
- Van de Vosse E., Bergen A. A., Meershoek E. J., Oosterwijk J. C., Gregory S., Bakker B., Weissenbach J., Coffey A. J., van Ommen G. J., Den Dunnen J. T. An Xp22.1-p22.2 YAC contig encompassing the disease loci for RS, KFSD, CLS, HYP and RP15: refined localization of RS. Eur J Hum Genet. 1996;4(2):101–104. doi: 10.1159/000472177. [DOI] [PubMed] [Google Scholar]
- Weber B. H., Janocha S., Vogt G., Sander S., Ewing C. C., Roesch M., Gibson A. X-linked juvenile retinoschisis (RS) maps between DXS987 and DXS443. Cytogenet Cell Genet. 1995;69(1-2):35–37. doi: 10.1159/000133932. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wieacker P., Wienker T. F., Dallapiccola B., Bender K., Davies K. E., Ropers H. H. Linkage relationships between Retinoschisis, Xg, and a cloned DNA sequence from the distal short arm of the X chromosome. Hum Genet. 1983;64(2):143–145. doi: 10.1007/BF00327111. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A. Disease gene mapping in isolated human populations: the example of Finland. J Med Genet. 1993 Oct;30(10):857–865. doi: 10.1136/jmg.30.10.857. [DOI] [PMC free article] [PubMed] [Google Scholar]


